Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fe(ClO4)3·H2O-Catalyzed Ritter Reaction: A Convenient Synthesis of Amides from Esters and Nitriles
Synlett ( IF 1.7 ) Pub Date : 2018-09-26 , DOI: 10.1055/s-0037-1610658 Min Ji 1 , Chengliang Feng 2 , Bin Yan 2 , Guibo Yin 2 , Junqing Chen 1
Synlett ( IF 1.7 ) Pub Date : 2018-09-26 , DOI: 10.1055/s-0037-1610658 Min Ji 1 , Chengliang Feng 2 , Bin Yan 2 , Guibo Yin 2 , Junqing Chen 1
Affiliation
|
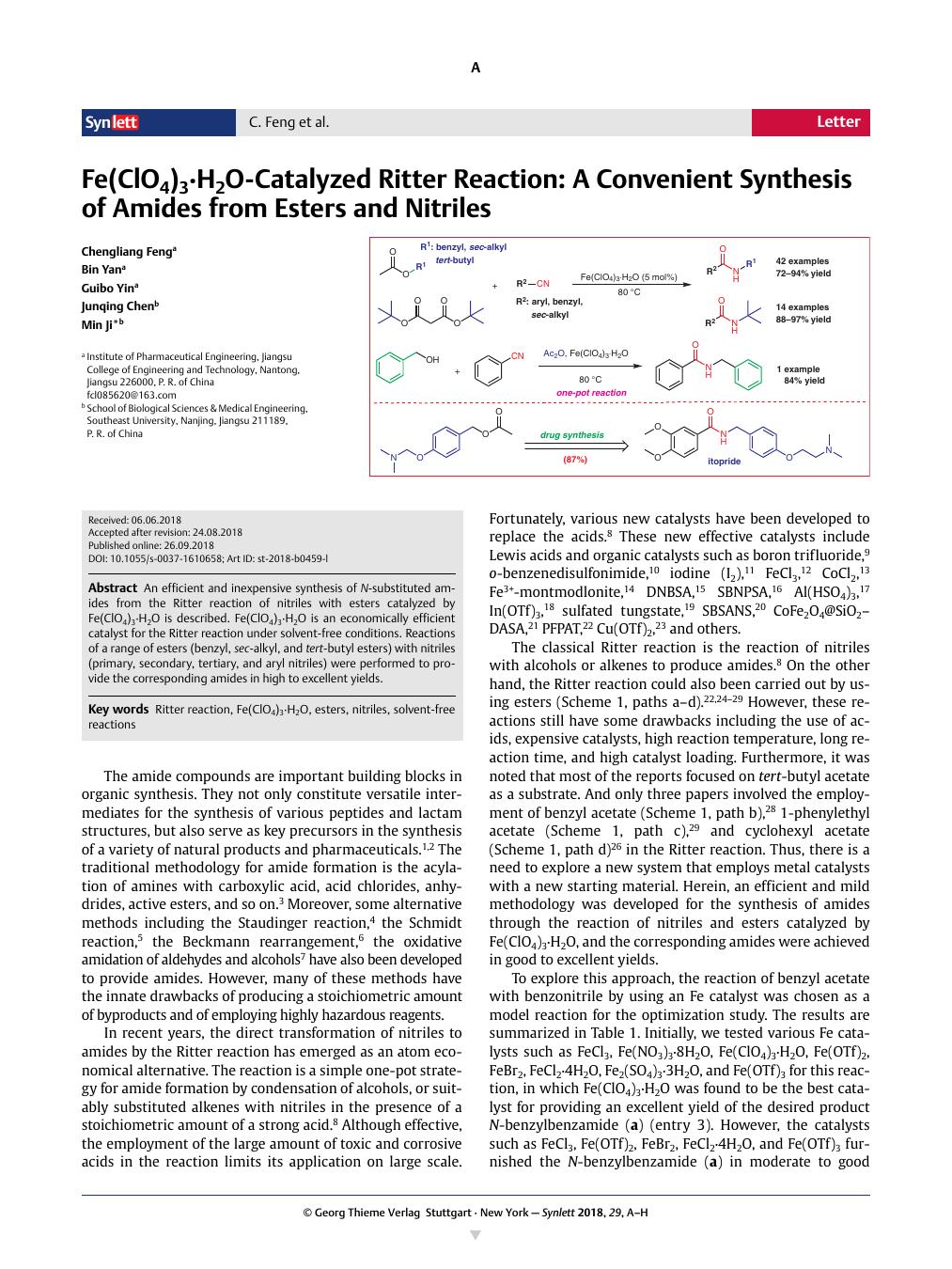
An efficient and inexpensive synthesis of N-substituted amides from the Ritter reaction of nitriles with esters catalyzed by Fe(ClO4)3·H2O is described. Fe(ClO4)3·H2O is an economically efficient catalyst for the Ritter reaction under solvent-free conditions. Reactions of a range of esters (benzyl, sec-alkyl, and tert-butyl esters) with nitriles (primary, secondary, tertiary, and aryl nitriles) were performed to provide the corresponding amides in high to excellent yields.
中文翻译:

Fe(ClO4)3·H2O-催化的里特反应:从酯和腈中方便地合成酰胺
描述了通过 Fe(ClO4)3·H2O 催化的腈与酯的 Ritter 反应高效且廉价地合成 N-取代酰胺。Fe(ClO4)3·H2O 是一种在无溶剂条件下进行 Ritter 反应的经济高效的催化剂。进行一系列酯(苄基、仲烷基和叔丁基酯)与腈(伯腈、仲腈、叔腈和芳基腈)的反应,以高产率至极好的产率提供相应的酰胺。
更新日期:2018-09-26
中文翻译:

Fe(ClO4)3·H2O-催化的里特反应:从酯和腈中方便地合成酰胺
描述了通过 Fe(ClO4)3·H2O 催化的腈与酯的 Ritter 反应高效且廉价地合成 N-取代酰胺。Fe(ClO4)3·H2O 是一种在无溶剂条件下进行 Ritter 反应的经济高效的催化剂。进行一系列酯(苄基、仲烷基和叔丁基酯)与腈(伯腈、仲腈、叔腈和芳基腈)的反应,以高产率至极好的产率提供相应的酰胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号