当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aminative Umpolung cyclization for synthesis of chiral exocyclic vicinal diamines
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-26 , DOI: 10.1039/c8ob02000k Feng Liu 1, 2, 3, 4 , Guoqing Zhao 1, 2, 3, 4 , Weiqi Cai 1, 2, 3, 4 , Dongfang Xu 1, 2, 3, 4 , Baoguo Zhao 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-26 , DOI: 10.1039/c8ob02000k Feng Liu 1, 2, 3, 4 , Guoqing Zhao 1, 2, 3, 4 , Weiqi Cai 1, 2, 3, 4 , Dongfang Xu 1, 2, 3, 4 , Baoguo Zhao 1, 2, 3, 4
Affiliation
|
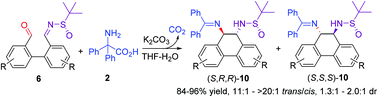
Chiral exocylic vicinal diamines are biologically and chemically important compounds, but they are not easy to make. In this paper, an interesting aminative Umpolung cyclization process has been developed. Aromatic aldehydes 6 bearing an electrophilic chiral sulfinimine group underwent imine formation with 2,2-diphenylglycine (2), decarboxylation, and subsequent Umpolung cyclization, producing various trans-diamines 10 in 84–96% yields with high trans/cis ratios under very mild conditions. This work not only provides an efficient, clean, and mild method for the synthesis of chiral exocyclic vicinal diamines in one step but also represents a new application of aminative Umpolung strategy on intramolecular reactions.
中文翻译:

胺Umpolung环化合成手性环外邻二胺
手性胞外邻二胺是生物学和化学上重要的化合物,但它们并不容易制造。在本文中,开发了一种有趣的Umpolung环化过程。带有亲电子手性亚氨基亚胺基团的芳香醛6经过2,2-二苯基甘氨酸(2)的亚胺形成,脱羧和随后的Umpolung环化反应,以84-96%的收率生产各种反式二胺10,具有高反式/顺式在非常温和的条件下的比率。这项工作不仅为一步合成手性环外邻二胺提供了一种高效,清洁,温和的方法,而且代表了胺化Umpolung策略在分子内反应中的新应用。
更新日期:2018-10-18
中文翻译:

胺Umpolung环化合成手性环外邻二胺
手性胞外邻二胺是生物学和化学上重要的化合物,但它们并不容易制造。在本文中,开发了一种有趣的Umpolung环化过程。带有亲电子手性亚氨基亚胺基团的芳香醛6经过2,2-二苯基甘氨酸(2)的亚胺形成,脱羧和随后的Umpolung环化反应,以84-96%的收率生产各种反式二胺10,具有高反式/顺式在非常温和的条件下的比率。这项工作不仅为一步合成手性环外邻二胺提供了一种高效,清洁,温和的方法,而且代表了胺化Umpolung策略在分子内反应中的新应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号