当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Secondary structures transition of tau protein with intrinsically disordered proteins specific force field.
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-10-17 , DOI: 10.1111/cbdd.13407 Aohuan Dan 1 , Hai-Feng Chen 1, 2
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-10-17 , DOI: 10.1111/cbdd.13407 Aohuan Dan 1 , Hai-Feng Chen 1, 2
Affiliation
|
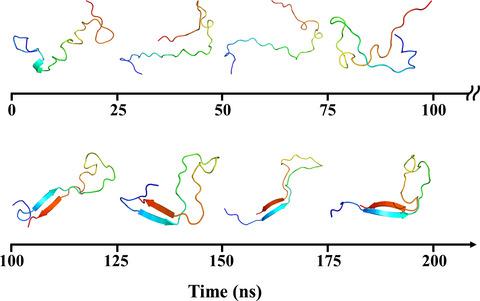
Microtubule-associated Tau protein plays a key role in assembling microtubule and modulating the functional organization of the neuron and developing axonal morphology, growth, and polarity. The pathological Tau can aggregate into cross-beta amyloid as one of the hallmarks for Alzheimer's disease (AD). Therefore, one of the top priorities in AD research is to figure out the structural model of Tau aggregation and to screen the inhibitors. The latest generation intrinsically disordered protein specific force field ff14IDPSFF significantly improved the distributions of heterogeneous conformations for intrinsically disordered proteins (IDPs). Here, the molecular dynamics (MD) simulations with three force fields of ff14SB, ff14IDPs, and ff14IDPSFF were employed to investigate the secondary structures transition of Tau (267-312) fragment. The results indicate that ff14IDPSFF can generate more heterogeneous conformers, and the predicted secondary structural distribution is closer to that of the experimental observation. In addition, predicted secondary chemical shifts from ff14IDPSFF are the most approach to those of experiment. Secondary structures transition kinetics for Tau(267-312) with ff14IDPSFF shows that the secondary structures were gradually transformed from α-helix to β-strand and the β-strand located at the regions of the residues 274-280 and residues 305-311. Besides, the driving force for the secondary structures transition of Tau(267-312) is mainly hydrophobic interactions which located at hexa-peptides 275 VQIINK280 and 306 VQIVYK311 . Secondary structure transition of Tau protein can give insight into the aggregation mechanism for AD.
中文翻译:

tau蛋白的二级结构转变具有固有的无序蛋白比力场。
微管相关的Tau蛋白在组装微管和调节神经元的功能组织以及发展轴突形态,生长和极性方面起着关键作用。病理性Tau可以聚集为跨β淀粉样蛋白,是阿尔茨海默氏病(AD)的标志之一。因此,AD研究的首要任务之一就是找出Tau聚集的结构模型并筛选抑制剂。最新一代的内在无序蛋白比力场ff14IDPSFF显着改善了内在无序蛋白(IDP)的异构构象分布。在这里,利用ff14SB,ff14IDPs和ff14IDPSFF三个力场的分子动力学(MD)模拟来研究Tau(267-312)片段的二级结构转变。结果表明ff14IDPSFF可以生成更多的异构构象,并且预测的二级结构分布更接近于实验观察的分布。此外,从ff14IDPSFF预测的第二化学位移是进行实验的最有效方法。带有ff14IDPSFF的Tau(267-312)的二级结构转变动力学表明,二级结构逐渐从α螺旋转变为β链,并且β链位于残基274-280和残基305-311的区域。此外,Tau(267-312)二级结构转变的驱动力主要是位于六肽275 VQIINK280和306 VQIVYK311上的疏水相互作用。Tau蛋白的二级结构转变可以深入了解AD的聚集机制。
更新日期:2018-10-17
中文翻译:

tau蛋白的二级结构转变具有固有的无序蛋白比力场。
微管相关的Tau蛋白在组装微管和调节神经元的功能组织以及发展轴突形态,生长和极性方面起着关键作用。病理性Tau可以聚集为跨β淀粉样蛋白,是阿尔茨海默氏病(AD)的标志之一。因此,AD研究的首要任务之一就是找出Tau聚集的结构模型并筛选抑制剂。最新一代的内在无序蛋白比力场ff14IDPSFF显着改善了内在无序蛋白(IDP)的异构构象分布。在这里,利用ff14SB,ff14IDPs和ff14IDPSFF三个力场的分子动力学(MD)模拟来研究Tau(267-312)片段的二级结构转变。结果表明ff14IDPSFF可以生成更多的异构构象,并且预测的二级结构分布更接近于实验观察的分布。此外,从ff14IDPSFF预测的第二化学位移是进行实验的最有效方法。带有ff14IDPSFF的Tau(267-312)的二级结构转变动力学表明,二级结构逐渐从α螺旋转变为β链,并且β链位于残基274-280和残基305-311的区域。此外,Tau(267-312)二级结构转变的驱动力主要是位于六肽275 VQIINK280和306 VQIVYK311上的疏水相互作用。Tau蛋白的二级结构转变可以深入了解AD的聚集机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号