当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Powering Motion with Enzymes
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-09-26 00:00:00 , DOI: 10.1021/acs.accounts.8b00286 Xi Zhao 1 , Kayla Gentile 1 , Farzad Mohajerani 2 , Ayusman Sen 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-09-26 00:00:00 , DOI: 10.1021/acs.accounts.8b00286 Xi Zhao 1 , Kayla Gentile 1 , Farzad Mohajerani 2 , Ayusman Sen 1
Affiliation
|
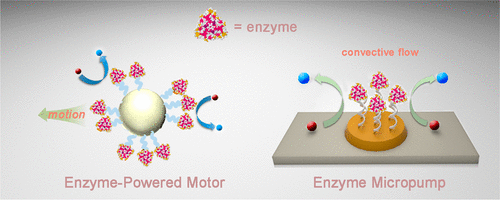
Enzymes are ubiquitous in living systems. Apart from traditional motor proteins, the function of enzymes was assumed to be confined to the promotion of biochemical reactions. Recent work shows that free swimming enzymes, when catalyzing reactions, generate enough mechanical force to cause their own movement, typically observed as substrate-concentration-dependent enhanced diffusion. Preliminary indication is that the impulsive force generated per turnover is comparable to the force produced by motor proteins and is within the range to activate biological adhesion molecules responsible for mechanosensation by cells, making force generation by enzymatic catalysis a novel mechanobiology-relevant event. Furthermore, when exposed to a gradient in substrate concentration, enzymes move up the gradient: an example of chemotaxis at the molecular level. The driving force for molecular chemotaxis appears to be the lowering of chemical potential due to thermodynamically favorable enzyme–substrate interactions and we suggest that chemotaxis promotes enzymatic catalysis by directing the motion of the catalyst and substrates toward each other.
中文翻译:

用酶为运动增添动力
酶在生命系统中无处不在。除了传统的运动蛋白,酶的功能被认为仅限于促进生化反应。最近的研究表明,游离的游泳酶在催化反应时会产生足够的机械力来引起自身的运动,通常以底物浓度依赖性的增强扩散作用来观察。初步迹象表明,每周转产生的冲力与运动蛋白产生的力相当,并且在激活负责细胞机械传感的生物粘附分子的范围内,使得通过酶催化产生的力成为一种新的与机械生物学相关的事件。此外,当暴露于底物浓度梯度时,酶会沿梯度向上移动:这是分子水平上趋化性的一个例子。
更新日期:2018-09-26
中文翻译:

用酶为运动增添动力
酶在生命系统中无处不在。除了传统的运动蛋白,酶的功能被认为仅限于促进生化反应。最近的研究表明,游离的游泳酶在催化反应时会产生足够的机械力来引起自身的运动,通常以底物浓度依赖性的增强扩散作用来观察。初步迹象表明,每周转产生的冲力与运动蛋白产生的力相当,并且在激活负责细胞机械传感的生物粘附分子的范围内,使得通过酶催化产生的力成为一种新的与机械生物学相关的事件。此外,当暴露于底物浓度梯度时,酶会沿梯度向上移动:这是分子水平上趋化性的一个例子。










































 京公网安备 11010802027423号
京公网安备 11010802027423号