Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanosensing by β1 integrin induces angiocrine signals for liver growth and survival
Nature ( IF 50.5 ) Pub Date : 2018-09-26 , DOI: 10.1038/s41586-018-0522-3 Linda Lorenz , Jennifer Axnick , Tobias Buschmann , Carina Henning , Sofia Urner , Shentong Fang , Harri Nurmi , Nicole Eichhorst , Richard Holtmeier , Kálmán Bódis , Jong-Hee Hwang , Karsten Müssig , Daniel Eberhard , Jörg Stypmann , Oliver Kuss , Michael Roden , Kari Alitalo , Dieter Häussinger , Eckhard Lammert
Nature ( IF 50.5 ) Pub Date : 2018-09-26 , DOI: 10.1038/s41586-018-0522-3 Linda Lorenz , Jennifer Axnick , Tobias Buschmann , Carina Henning , Sofia Urner , Shentong Fang , Harri Nurmi , Nicole Eichhorst , Richard Holtmeier , Kálmán Bódis , Jong-Hee Hwang , Karsten Müssig , Daniel Eberhard , Jörg Stypmann , Oliver Kuss , Michael Roden , Kari Alitalo , Dieter Häussinger , Eckhard Lammert
|
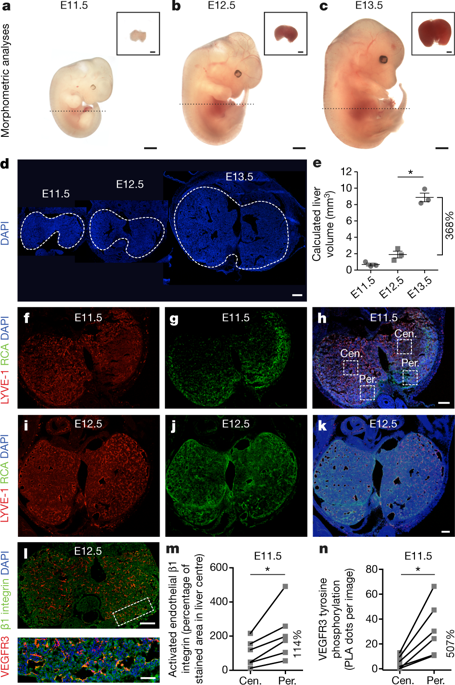
Angiocrine signals derived from endothelial cells are an important component of intercellular communication and have a key role in organ growth, regeneration and disease1–4. These signals have been identified and studied in multiple organs, including the liver, pancreas, lung, heart, bone, bone marrow, central nervous system, retina and some cancers1–4. Here we use the developing liver as a model organ to study angiocrine signals5,6, and show that the growth rate of the liver correlates both spatially and temporally with blood perfusion to this organ. By manipulating blood flow through the liver vasculature, we demonstrate that vessel perfusion activates β1 integrin and vascular endothelial growth factor receptor 3 (VEGFR3). Notably, both β1 integrin and VEGFR3 are strictly required for normal production of hepatocyte growth factor, survival of hepatocytes and liver growth. Ex vivo perfusion of adult mouse liver and in vitro mechanical stretching of human hepatic endothelial cells illustrate that mechanotransduction alone is sufficient to turn on angiocrine signals. When the endothelial cells are mechanically stretched, angiocrine signals trigger in vitro proliferation and survival of primary human hepatocytes. Our findings uncover a signalling pathway in vascular endothelial cells that translates blood perfusion and mechanotransduction into organ growth and maintenance.In mouse and human liver models, blood vessel perfusion and mechanical stretching release angiocrine signals from endothelial cells that lead to hepatocyte survival and liver growth.
中文翻译:

β1 整合素的机械感应诱导肝脏生长和存活的血管分泌信号
来自内皮细胞的血管分泌信号是细胞间通讯的重要组成部分,在器官生长、再生和疾病中起着关键作用1-4。这些信号已在多个器官中得到识别和研究,包括肝脏、胰腺、肺、心脏、骨骼、骨髓、中枢神经系统、视网膜和一些癌症1-4。在这里,我们使用发育中的肝脏作为模型器官来研究血管分泌信号 5,6,并表明肝脏的生长速度在空间和时间上都与该器官的血液灌注相关。通过操纵通过肝脏血管系统的血流,我们证明血管灌注激活 β1 整合素和血管内皮生长因子受体 3 (VEGFR3)。值得注意的是,β1 整合素和 VEGFR3 都是肝细胞生长因子正常产生所必需的,肝细胞存活和肝脏生长。成年小鼠肝脏的离体灌注和人肝内皮细胞的体外机械拉伸说明单独的机械转导足以打开血管分泌信号。当内皮细胞受到机械拉伸时,血管分泌信号会触发原代人肝细胞的体外增殖和存活。我们的研究结果揭示了血管内皮细胞中的信号通路,该通路将血液灌注和机械转导转化为器官生长和维持。在小鼠和人类肝脏模型中,血管灌注和机械拉伸从内皮细胞释放血管分泌信号,导致肝细胞存活和肝脏生长。成年小鼠肝脏的离体灌注和人肝内皮细胞的体外机械拉伸说明单独的机械转导足以打开血管分泌信号。当内皮细胞受到机械拉伸时,血管分泌信号会触发原代人肝细胞的体外增殖和存活。我们的研究结果揭示了血管内皮细胞中的信号通路,该通路将血液灌注和机械转导转化为器官生长和维持。在小鼠和人类肝脏模型中,血管灌注和机械拉伸从内皮细胞释放血管分泌信号,导致肝细胞存活和肝脏生长。成年小鼠肝脏的离体灌注和人肝内皮细胞的体外机械拉伸说明单独的机械转导足以打开血管分泌信号。当内皮细胞受到机械拉伸时,血管分泌信号会触发原代人肝细胞的体外增殖和存活。我们的研究结果揭示了血管内皮细胞中的信号通路,该通路将血液灌注和机械转导转化为器官生长和维持。在小鼠和人类肝脏模型中,血管灌注和机械拉伸从内皮细胞释放血管分泌信号,导致肝细胞存活和肝脏生长。血管分泌信号触发原代人肝细胞的体外增殖和存活。我们的研究结果揭示了血管内皮细胞中的信号通路,该通路将血液灌注和机械转导转化为器官生长和维持。在小鼠和人类肝脏模型中,血管灌注和机械拉伸从内皮细胞释放血管分泌信号,导致肝细胞存活和肝脏生长。血管分泌信号触发原代人肝细胞的体外增殖和存活。我们的研究结果揭示了血管内皮细胞中的信号通路,该通路将血液灌注和机械转导转化为器官生长和维持。在小鼠和人类肝脏模型中,血管灌注和机械拉伸从内皮细胞释放血管分泌信号,导致肝细胞存活和肝脏生长。
更新日期:2018-09-26
中文翻译:

β1 整合素的机械感应诱导肝脏生长和存活的血管分泌信号
来自内皮细胞的血管分泌信号是细胞间通讯的重要组成部分,在器官生长、再生和疾病中起着关键作用1-4。这些信号已在多个器官中得到识别和研究,包括肝脏、胰腺、肺、心脏、骨骼、骨髓、中枢神经系统、视网膜和一些癌症1-4。在这里,我们使用发育中的肝脏作为模型器官来研究血管分泌信号 5,6,并表明肝脏的生长速度在空间和时间上都与该器官的血液灌注相关。通过操纵通过肝脏血管系统的血流,我们证明血管灌注激活 β1 整合素和血管内皮生长因子受体 3 (VEGFR3)。值得注意的是,β1 整合素和 VEGFR3 都是肝细胞生长因子正常产生所必需的,肝细胞存活和肝脏生长。成年小鼠肝脏的离体灌注和人肝内皮细胞的体外机械拉伸说明单独的机械转导足以打开血管分泌信号。当内皮细胞受到机械拉伸时,血管分泌信号会触发原代人肝细胞的体外增殖和存活。我们的研究结果揭示了血管内皮细胞中的信号通路,该通路将血液灌注和机械转导转化为器官生长和维持。在小鼠和人类肝脏模型中,血管灌注和机械拉伸从内皮细胞释放血管分泌信号,导致肝细胞存活和肝脏生长。成年小鼠肝脏的离体灌注和人肝内皮细胞的体外机械拉伸说明单独的机械转导足以打开血管分泌信号。当内皮细胞受到机械拉伸时,血管分泌信号会触发原代人肝细胞的体外增殖和存活。我们的研究结果揭示了血管内皮细胞中的信号通路,该通路将血液灌注和机械转导转化为器官生长和维持。在小鼠和人类肝脏模型中,血管灌注和机械拉伸从内皮细胞释放血管分泌信号,导致肝细胞存活和肝脏生长。成年小鼠肝脏的离体灌注和人肝内皮细胞的体外机械拉伸说明单独的机械转导足以打开血管分泌信号。当内皮细胞受到机械拉伸时,血管分泌信号会触发原代人肝细胞的体外增殖和存活。我们的研究结果揭示了血管内皮细胞中的信号通路,该通路将血液灌注和机械转导转化为器官生长和维持。在小鼠和人类肝脏模型中,血管灌注和机械拉伸从内皮细胞释放血管分泌信号,导致肝细胞存活和肝脏生长。血管分泌信号触发原代人肝细胞的体外增殖和存活。我们的研究结果揭示了血管内皮细胞中的信号通路,该通路将血液灌注和机械转导转化为器官生长和维持。在小鼠和人类肝脏模型中,血管灌注和机械拉伸从内皮细胞释放血管分泌信号,导致肝细胞存活和肝脏生长。血管分泌信号触发原代人肝细胞的体外增殖和存活。我们的研究结果揭示了血管内皮细胞中的信号通路,该通路将血液灌注和机械转导转化为器官生长和维持。在小鼠和人类肝脏模型中,血管灌注和机械拉伸从内皮细胞释放血管分泌信号,导致肝细胞存活和肝脏生长。











































 京公网安备 11010802027423号
京公网安备 11010802027423号