当前位置:
X-MOL 学术
›
Acta Pharmacol. Sin.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway.
Acta Pharmacologica Sinica ( IF 6.9 ) Pub Date : 2018-09-27 , DOI: 10.1038/s41401-018-0154-z Shi-Feng Chu 1 , Zhao Zhang 1 , Xin Zhou 1 , Wen-Bin He 1, 2 , Chen Chen 1 , Piao Luo 3 , Dan-Dan Liu 1 , Qi-di Ai 3 , Hai-Fan Gong 4 , Zhen-Zhen Wang 1 , Hong-Shuo Sun 4 , Zhong-Ping Feng 4 , Nai-Hong Chen 1, 3
Acta Pharmacologica Sinica ( IF 6.9 ) Pub Date : 2018-09-27 , DOI: 10.1038/s41401-018-0154-z Shi-Feng Chu 1 , Zhao Zhang 1 , Xin Zhou 1 , Wen-Bin He 1, 2 , Chen Chen 1 , Piao Luo 3 , Dan-Dan Liu 1 , Qi-di Ai 3 , Hai-Fan Gong 4 , Zhen-Zhen Wang 1 , Hong-Shuo Sun 4 , Zhong-Ping Feng 4 , Nai-Hong Chen 1, 3
Affiliation
|
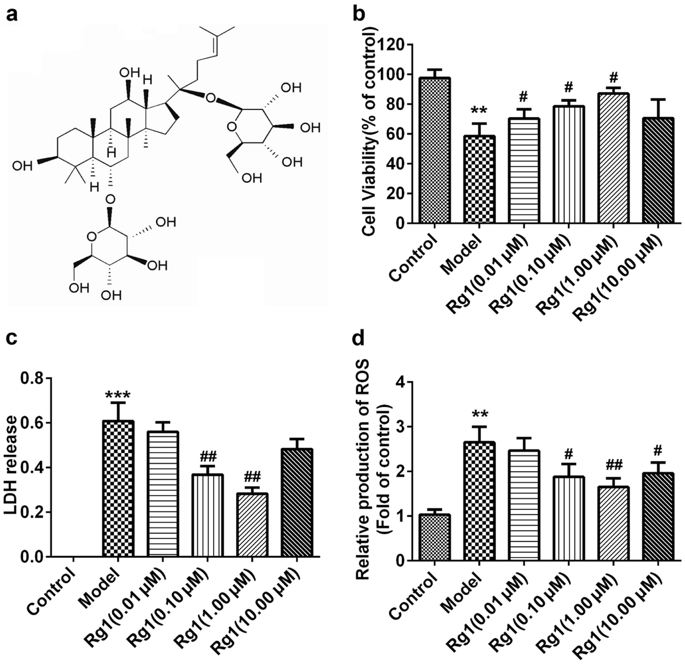
Ginsenoside Rg1 (Rg1), a saponin extracted from Panax ginseng, has been well documented to be effective against ischemic/reperfusion (I/R) neuronal injury. However, the underlying mechanisms remain obscure. In the present study, we investigated the roles of Nrf2 and miR-144 in the protective effects of Rg1 against I/R-induced neuronal injury. In OGD/R-treated PC12 cells, Rg1 (0.01-1 μmol/L) dose-dependently attenuated the cell injury accompanied by prolonging nuclear accumulation of Nrf2, enhancing the transcriptional activity of Nrf2, as well as promoting the expression of ARE-target genes. The activation of the Nrf2/ARE pathway by Rg1 was independent of disassociation with Keap1, but resulted from post-translational regulations. Knockdown of Nrf2 abolished all the protective changes of Rg1 in OGD/R-treated PC12 cells. Furthermore, Rg1 treatment significantly decreased the expression of miR-144, which downregulated Nrf2 production by targeting its 3'-untranlated region after OGD/R. Knockdown of Nrf2 had no effect on the expression of miR-144, suggesting that miR-144 was an upstream regulator of Nrf2. We revealed that there was a direct binding between Nrf2 and miR-144 in PC12 cells. Application of anti-miR-144 occluded the activation of the Nrf2/ARE pathway by Rg1 in OGD/R-treated PC12 cells. In tMCAO rats, administration of Rg1 (20 mg/kg) significantly alleviated ischemic injury, and activated Nrf2/ARE pathway. The protective effects of Rg1 were abolished by injecting of AAV-HIF-miR-144-shRNA into the predicted ischemic penumbra. In conclusion, our results demonstrate that Rg1 alleviates oxidative stress after I/R through inhibiting miR-144 activity and subsequently promoting the Nrf2/ARE pathway at the post-translational level.
中文翻译:

人参皂苷 Rg1 通过 miR-144/Nrf2/ARE 途径防止缺血/再灌注引起的神经元损伤。
人参皂苷 Rg1 (Rg1) 是一种从人参中提取的皂苷,已被充分证明可有效对抗缺血/再灌注 (I/R) 神经元损伤。然而,潜在的机制仍然不清楚。在本研究中,我们研究了 Nrf2 和 miR-144 在 Rg1 对 I/R 诱导的神经元损伤的保护作用中的作用。在 OGD/R 处理的 PC12 细胞中,Rg1(0.01-1 μmol/L)剂量依赖性地减轻细胞损伤,同时延长 Nrf2 的核积累,增强 Nrf2 的转录活性,并促进 ARE-target 的表达基因。 Rg1 对 Nrf2/ARE 通路的激活与 Keap1 的解离无关,而是由翻译后调节引起的。 Nrf2 的敲低消除了 OGD/R 处理的 PC12 细胞中 Rg1 的所有保护性变化。此外,Rg1 处理显着降低了 miR-144 的表达,在 OGD/R 后,miR-144 通过靶向其 3'-非翻译区来下调 Nrf2 的产生。 Nrf2 的敲低对 miR-144 的表达没有影响,表明 miR-144 是 Nrf2 的上游调节因子。我们发现 PC12 细胞中 Nrf2 和 miR-144 之间存在直接结合。在 OGD/R 处理的 PC12 细胞中,应用抗 miR-144 阻断了 Rg1 对 Nrf2/ARE 通路的激活。在 tMCAO 大鼠中,给予 Rg1(20 mg/kg)可显着减轻缺血性损伤,并激活 Nrf2/ARE 通路。通过将 AAV-HIF-miR-144-shRNA 注射到预测的缺血半影中,Rg1 的保护作用被消除。总之,我们的结果表明,Rg1 通过抑制 miR-144 活性并随后在翻译后水平促进 Nrf2/ARE 通路来减轻 I/R 后的氧化应激。
更新日期:2019-01-26
中文翻译:

人参皂苷 Rg1 通过 miR-144/Nrf2/ARE 途径防止缺血/再灌注引起的神经元损伤。
人参皂苷 Rg1 (Rg1) 是一种从人参中提取的皂苷,已被充分证明可有效对抗缺血/再灌注 (I/R) 神经元损伤。然而,潜在的机制仍然不清楚。在本研究中,我们研究了 Nrf2 和 miR-144 在 Rg1 对 I/R 诱导的神经元损伤的保护作用中的作用。在 OGD/R 处理的 PC12 细胞中,Rg1(0.01-1 μmol/L)剂量依赖性地减轻细胞损伤,同时延长 Nrf2 的核积累,增强 Nrf2 的转录活性,并促进 ARE-target 的表达基因。 Rg1 对 Nrf2/ARE 通路的激活与 Keap1 的解离无关,而是由翻译后调节引起的。 Nrf2 的敲低消除了 OGD/R 处理的 PC12 细胞中 Rg1 的所有保护性变化。此外,Rg1 处理显着降低了 miR-144 的表达,在 OGD/R 后,miR-144 通过靶向其 3'-非翻译区来下调 Nrf2 的产生。 Nrf2 的敲低对 miR-144 的表达没有影响,表明 miR-144 是 Nrf2 的上游调节因子。我们发现 PC12 细胞中 Nrf2 和 miR-144 之间存在直接结合。在 OGD/R 处理的 PC12 细胞中,应用抗 miR-144 阻断了 Rg1 对 Nrf2/ARE 通路的激活。在 tMCAO 大鼠中,给予 Rg1(20 mg/kg)可显着减轻缺血性损伤,并激活 Nrf2/ARE 通路。通过将 AAV-HIF-miR-144-shRNA 注射到预测的缺血半影中,Rg1 的保护作用被消除。总之,我们的结果表明,Rg1 通过抑制 miR-144 活性并随后在翻译后水平促进 Nrf2/ARE 通路来减轻 I/R 后的氧化应激。











































 京公网安备 11010802027423号
京公网安备 11010802027423号