当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Brain Penetrating Bifunctional Erythropoietin–Transferrin Receptor Antibody Fusion Protein for Alzheimer’s Disease
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-09-25 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00594 Rudy Chang 1 , Abrar Al Maghribi 2 , Victoria Vanderpoel 3 , Vitaly Vasilevko 4 , David H. Cribbs 4 , Ruben Boado 5 , William M. Pardridge 5 , Rachita K. Sumbria 1, 4
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-09-25 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00594 Rudy Chang 1 , Abrar Al Maghribi 2 , Victoria Vanderpoel 3 , Vitaly Vasilevko 4 , David H. Cribbs 4 , Ruben Boado 5 , William M. Pardridge 5 , Rachita K. Sumbria 1, 4
Affiliation
|
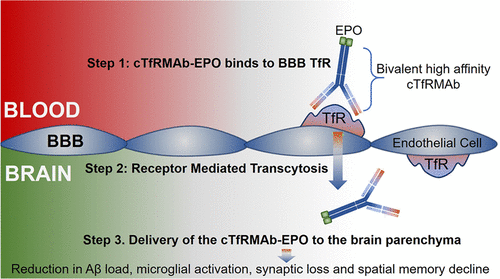
Erythropoietin (EPO), a glycoprotein cytokine essential to hematopoiesis, has neuroprotective effects in rodent models of Alzheimer’s disease (AD). However, high therapeutic doses or invasive routes of administration of EPO are required to achieve effective brain concentrations due to low blood–brain barrier (BBB) penetrability, and high EPO doses result in hematopoietic side effects. These obstacles can be overcome by engineering a BBB-penetrable analog of EPO, which is rapidly cleared from the blood, by fusing EPO to a chimeric monoclonal antibody targeting the transferrin receptor (cTfRMAb), which acts as a molecular Trojan horse to ferry the EPO into the brain via the transvascular route. In the current study, we investigated the effects of the BBB-penetrable analog of EPO on AD pathology in a double transgenic mouse model of AD. Five and a half month old male APPswe/PSEN1dE9 (APP/PS1) transgenic mice were treated with saline (n = 10) or the BBB-penetrable EPO (n = 10) 3 days/week intraperitoneally for 8 weeks, compared to same-aged C57BL/6J wild-type mice treated with saline (n = 8) with identical regiment. At 9 weeks following treatment initiation, exploration and spatial memory were assessed with the open-field and Y-maze test, mice were sacrificed, and brains were evaluated for Aβ peptide load, synaptic loss, BBB disruption, microglial activation, and microhemorrhages. APP/PS1 mice treated with the BBB-penetrable cTfRMAb-EPO fusion protein had significantly lower cortical and hippocampal Aβ peptide number (p < 0.05) and immune-positive area (p < 0.05), a decrease in hippocampal synaptic loss (p < 0.05) and cortical microglial activation (p < 0.001), and improved spatial memory (p < 0.05) compared with APP/PS1 saline controls. BBB-penetrating EPO was not associated with microhemorrhage development. The cTfRMAb-EPO fusion protein offers therapeutic benefits by targeting multiple targets of AD pathogenesis and progression (Aβ load, synaptic loss, microglial activation) and improving spatial memory in the APP/PS1 mouse model of AD.
中文翻译:

脑穿透性双功能促红细胞生成素-转铁蛋白受体抗体融合蛋白用于阿尔茨海默氏病
促红细胞生成素(EPO)是造血作用所必需的糖蛋白细胞因子,在阿尔茨海默氏病(AD)的啮齿动物模型中具有神经保护作用。然而,由于血脑屏障(BBB)的低渗透性,需要高剂量的治疗药物或侵入性的EPO途径才能达到有效的大脑浓度,而高剂量的EPO会导致造血副作用。通过将EPO与靶向转铁蛋白受体的嵌合单克隆抗体(cTfRMAb)融合,可工程化从血液中快速清除的BBB可穿透的EPO类似物,从而克服这些障碍,该分子充当特洛伊木马分子运送EPO通过血管途径进入大脑。在当前的研究中,我们研究了双转基因AD小鼠模型中EPO的BBB可穿透类似物对AD病理的影响。n = 10)或BBB穿透性EPO(n = 10)腹膜内注射3天/周,连续8周,与相同剂量的生理盐水(n = 8)治疗的同龄C57BL / 6J野生型小鼠相比。治疗开始后第9周,通过野外和Y迷宫测试评估探索和空间记忆,处死小鼠,并评估大脑的Aβ肽负荷,突触丧失,BBB破坏,小胶质细胞活化和微出血。用可穿透BBB的cTfRMAb-EPO融合蛋白处理的APP / PS1小鼠的皮质和海马Aβ肽数量明显减少(p <0.05)和免疫阳性区域(p <0.05),海马突触损失减少(p与APP / PS1盐水对照组相比,<0.05)和皮质小胶质细胞激活(p <0.001),以及改善的空间记忆(p <0.05)。穿透BBB的EPO与微出血的发展无关。cTfRMAb-EPO融合蛋白通过靶向AD发病机理和进展的多个靶点(Aβ负荷,突触丧失,小胶质细胞活化)并改善AD的APP / PS1小鼠模型的空间记忆而提供治疗益处。
更新日期:2018-09-25
中文翻译:

脑穿透性双功能促红细胞生成素-转铁蛋白受体抗体融合蛋白用于阿尔茨海默氏病
促红细胞生成素(EPO)是造血作用所必需的糖蛋白细胞因子,在阿尔茨海默氏病(AD)的啮齿动物模型中具有神经保护作用。然而,由于血脑屏障(BBB)的低渗透性,需要高剂量的治疗药物或侵入性的EPO途径才能达到有效的大脑浓度,而高剂量的EPO会导致造血副作用。通过将EPO与靶向转铁蛋白受体的嵌合单克隆抗体(cTfRMAb)融合,可工程化从血液中快速清除的BBB可穿透的EPO类似物,从而克服这些障碍,该分子充当特洛伊木马分子运送EPO通过血管途径进入大脑。在当前的研究中,我们研究了双转基因AD小鼠模型中EPO的BBB可穿透类似物对AD病理的影响。n = 10)或BBB穿透性EPO(n = 10)腹膜内注射3天/周,连续8周,与相同剂量的生理盐水(n = 8)治疗的同龄C57BL / 6J野生型小鼠相比。治疗开始后第9周,通过野外和Y迷宫测试评估探索和空间记忆,处死小鼠,并评估大脑的Aβ肽负荷,突触丧失,BBB破坏,小胶质细胞活化和微出血。用可穿透BBB的cTfRMAb-EPO融合蛋白处理的APP / PS1小鼠的皮质和海马Aβ肽数量明显减少(p <0.05)和免疫阳性区域(p <0.05),海马突触损失减少(p与APP / PS1盐水对照组相比,<0.05)和皮质小胶质细胞激活(p <0.001),以及改善的空间记忆(p <0.05)。穿透BBB的EPO与微出血的发展无关。cTfRMAb-EPO融合蛋白通过靶向AD发病机理和进展的多个靶点(Aβ负荷,突触丧失,小胶质细胞活化)并改善AD的APP / PS1小鼠模型的空间记忆而提供治疗益处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号