Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-09-25 , DOI: 10.1016/j.tetlet.2018.09.059 Ming-hao Wu , Yu-lei Li , Qi Chang , Xia Zhao , Qing Chen
|
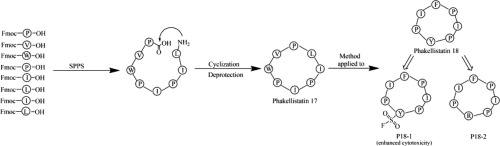
The Phakellistatins 17 and 18, two naturally occurring cyclic proline-rich peptides from marine sponge, were synthesized by utilizing a two-step solid-phase/solution synthesis strategy for the first time. Phakellistatin 18 exhibited a weak inhibitory activity against human lung carcinoma cell (A549) and human hepatoma (BEL-7042). In order to improve the activity of Phakellistatin 18, two of its analogues which contain sulfonyl fluoride group (P18-1) and arginine substituted derivative (P18-2) were also synthesized respectively. The spectral data of Phakellistatins 17 and 18 were identical to that reported for the natural products. Analogues P18-1 and P18-2 were identified by means of HR-QTOF-MS, 1H NMR and 13C NMR. More importantly, analogue P18-1 exhibited significantly enhanced cytotoxicity against BEL-7042 cancer cells compared with Phakellistatin 18, suggesting that the sulfonyl fluoride group in the parent peptide may contribute to the improvement of antitumor activity. This strategy provides a reference method for improving the activity of natural peptides. reserved.
中文翻译:

从海洋海绵中分离出的富含脯氨酸的环肽Phakellistatins 17和18的全合成和修饰
Phakellistatins 17和18是两种来自海洋海绵的天然存在的富含环脯氨酸的肽,是首次通过两步固相/溶液合成策略合成的。伐他汀18对人肺癌细胞(A549)和人肝癌(BEL-7042)的抑制作用较弱。为了提高Phakellistatin 18的活性,还分别合成了两个含有磺酰氟基团(P18-1)和精氨酸取代衍生物(P18-2)的类似物。Phakellistatins 17和18的光谱数据与报道的天然产物相同。通过HR-QTOF-MS,1 H NMR和13鉴定类似物P18-1和P18-21 H NMR。更重要的是,与Phakellistatin 18相比,类似物P18-1对BEL-7042癌细胞表现出明显增强的细胞毒性,这表明母体肽中的磺酰氟基团可能有助于提高抗肿瘤活性。该策略为提高天然肽的活性提供了参考方法。预订的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号