当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gas-Phase Chemistry of the NO–SO2–ClO2 System Applied to Flue Gas Cleaning
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-10-17 , DOI: 10.1021/acs.iecr.8b03067 Jakob Johansson 1 , Anette Heijnesson Hultén 2 , Sima Ajdari 1 , Pär Nilsson 2 , Marie Samuelsson 2 , Fredrik Normann 1 , Klas Andersson 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-10-17 , DOI: 10.1021/acs.iecr.8b03067 Jakob Johansson 1 , Anette Heijnesson Hultén 2 , Sima Ajdari 1 , Pär Nilsson 2 , Marie Samuelsson 2 , Fredrik Normann 1 , Klas Andersson 1
Affiliation
|
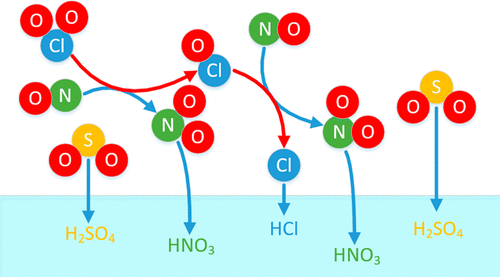
The chemical interactions that occur between NO, SO2, and ClO2 are investigated. In focus is the oxidation of NO with gaseous ClO2 for simultaneous removal of NOx and SOx from combustion-derived flue gases. Laboratory-scale experiments were conducted to examine the conversion of NO to NO2, under the following conditions: temperature range of 100–180 °C; H2O concentrations in the range of 0%–25%; ClO2–NO molar ratios in the range of 0.2–0.6; and NO and SO2 flue gas concentrations in the ranges of 0–250 ppm and 0–1000 ppm, respectively. The results show that NO is oxidized efficiently by ClO2, whereas the ClO2–SO2 reactions are insignificant. The water concentration had no effect on oxidation, and the temperature had a limited effect, within the investigated ranges. The outcomes favor the process economics of the studied application, since ClO2 consumption is negligible with respect to SO2 oxidation.
中文翻译:

NO–SO 2 –ClO 2系统的气相化学应用于烟气净化
研究了NO,SO 2和ClO 2之间发生的化学相互作用。重点是用气态ClO 2氧化NO,以同时从燃烧烟道气中去除NO x和SO x。在以下条件下进行了实验室规模的实验,以检查NO向NO 2的转化:温度范围为100–180°C;H 2 O的浓度范围为0%–25%;ClO 2 -NO摩尔比在0.2-0.6范围内;NO和SO 2的烟气浓度分别在0–250 ppm和0–1000 ppm的范围内。结果表明,NO被ClO 2有效氧化,而ClO 2 -SO 2反应无关紧要。在所研究的范围内,水的浓度对氧化没有影响,并且温度的影响有限。该结果有利于所研究应用的过程经济学,因为就SO 2氧化而言,ClO 2的消耗可忽略不计。
更新日期:2018-10-18
中文翻译:

NO–SO 2 –ClO 2系统的气相化学应用于烟气净化
研究了NO,SO 2和ClO 2之间发生的化学相互作用。重点是用气态ClO 2氧化NO,以同时从燃烧烟道气中去除NO x和SO x。在以下条件下进行了实验室规模的实验,以检查NO向NO 2的转化:温度范围为100–180°C;H 2 O的浓度范围为0%–25%;ClO 2 -NO摩尔比在0.2-0.6范围内;NO和SO 2的烟气浓度分别在0–250 ppm和0–1000 ppm的范围内。结果表明,NO被ClO 2有效氧化,而ClO 2 -SO 2反应无关紧要。在所研究的范围内,水的浓度对氧化没有影响,并且温度的影响有限。该结果有利于所研究应用的过程经济学,因为就SO 2氧化而言,ClO 2的消耗可忽略不计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号