当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
B(C6F5)3/Amine‐Catalyzed C(sp)−H Silylation of Terminal Alkynes with Hydrosilanes: Experimental and Theoretical Studies
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-17 , DOI: 10.1002/anie.201809533 Yuanhong Ma 1 , Shao‐Jie Lou 2 , Gen Luo 3 , Yong Luo 1 , Gu Zhan 1 , Masayoshi Nishiura 1, 2 , Yi Luo 3 , Zhaomin Hou 1, 2, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-17 , DOI: 10.1002/anie.201809533 Yuanhong Ma 1 , Shao‐Jie Lou 2 , Gen Luo 3 , Yong Luo 1 , Gu Zhan 1 , Masayoshi Nishiura 1, 2 , Yi Luo 3 , Zhaomin Hou 1, 2, 3
Affiliation
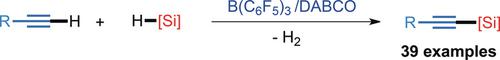
|
Transition metal catalyzed C−H functionalization of organic compounds has proved to be a useful atom‐efficient strategy in organic synthesis. In contrast, main‐group‐element‐based catalytic processes for C−H functionalization have remained underexplored to date. Reported herein is the catalytic C(sp)−H silylation of a wide range of terminal alkynes with hydrosilanes by using a combination of B(C6F5)3 and an organic base such as triethylenediamine (DABCO). This protocol constitutes the first example of boron‐catalyzed C(sp)−H functionalization, offering a convenient route for the synthesis of a variety of alkynylsilanes. Experimental and computational studies have revealed that DABCO plays two crucial roles (Lewis base and Brønsted base) in this catalytic transformation.
中文翻译:

B(C6F5)3 /胺催化末端炔烃与氢硅烷的C(sp)-H硅烷化:实验和理论研究
过渡金属催化的有机化合物的CH官能化已被证明是有机合成中有用的原子效率策略。相比之下,迄今为止,对于CHH功能化的基于主族元素的催化过程仍未得到充分研究。本文报道的是通过使用B(C 6 F 5)3的组合,各种末端炔烃与氢硅烷的催化C(sp)-H甲硅烷基化反应和有机碱,例如三乙二胺(DABCO)。该协议构成了硼催化的C(sp)-H功能化的第一个例子,为合成各种炔基硅烷提供了一条便捷的途径。实验和计算研究表明,DABCO在此催化转化中起着两个至关重要的作用(刘易斯碱和布朗斯台德碱)。
更新日期:2018-10-17
中文翻译:

B(C6F5)3 /胺催化末端炔烃与氢硅烷的C(sp)-H硅烷化:实验和理论研究
过渡金属催化的有机化合物的CH官能化已被证明是有机合成中有用的原子效率策略。相比之下,迄今为止,对于CHH功能化的基于主族元素的催化过程仍未得到充分研究。本文报道的是通过使用B(C 6 F 5)3的组合,各种末端炔烃与氢硅烷的催化C(sp)-H甲硅烷基化反应和有机碱,例如三乙二胺(DABCO)。该协议构成了硼催化的C(sp)-H功能化的第一个例子,为合成各种炔基硅烷提供了一条便捷的途径。实验和计算研究表明,DABCO在此催化转化中起着两个至关重要的作用(刘易斯碱和布朗斯台德碱)。










































 京公网安备 11010802027423号
京公网安备 11010802027423号