当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Processive Nanostepping of Formin mDia1 Loosely Coupled with Actin Polymerization
Nano Letters ( IF 9.6 ) Pub Date : 2018-09-25 00:00:00 , DOI: 10.1021/acs.nanolett.8b03277 Hiroaki Kubota 1 , Makito Miyazaki 1 , Taisaku Ogawa 1 , Togo Shimozawa 2 , Kazuhiko Kinosita 1 , Shin’ichi Ishiwata 1
Nano Letters ( IF 9.6 ) Pub Date : 2018-09-25 00:00:00 , DOI: 10.1021/acs.nanolett.8b03277 Hiroaki Kubota 1 , Makito Miyazaki 1 , Taisaku Ogawa 1 , Togo Shimozawa 2 , Kazuhiko Kinosita 1 , Shin’ichi Ishiwata 1
Affiliation
|
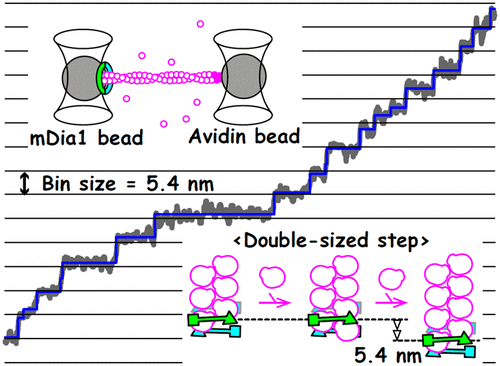
Formins are actin-binding proteins that construct nanoscale machinery with the growing barbed end of actin filaments and serve as key regulators of actin polymerization and depolymerization. To maintain the regulation of actin dynamics, formins have been proposed to processively move at every association or dissociation of a single actin molecule toward newly formed barbed ends. However, the current models for the motile mechanisms were established without direct observation of the elementary processes of this movement. Here, using optical tweezers, we demonstrate that formin mDia1 moves stepwise, observed at a nanometer spatial resolution. The movement was composed of forward and backward steps with unitary step sizes of 2.8 and −2.4 nm, respectively, which nearly equaled the actin subunit length (∼2.7 nm), consistent with the generally accepted models. However, in addition to steps equivalent to the length of a single actin subunit, those equivalent to the length of two or three subunits were frequently observed. Our findings suggest that the coupling between mDia1 stepping and actin polymerization is not tight but loose, which may be achieved by the multiple binding states of mDia1, providing insights into the synergistic functions of biomolecules for the efficient construction and regulation of nanofilaments.
中文翻译:

松散耦合肌动蛋白聚合的Formin mDia1的逐步纳米步骤。
Formin是肌动蛋白结合蛋白,可构成具有肌动蛋白丝长刺末端的纳米级机器,并充当肌动蛋白聚合和解聚的关键调节剂。为了维持肌动蛋白动力学的调节,已经提出了福明蛋白在单个肌动蛋白分子的每次缔合或解离处向新形成的带刺末端进行性移动。但是,建立运动机制的当前模型时没有直接观察到该运动的基本过程。在这里,使用光镊,我们证明了以纳米空间分辨率观察到的formin mDia1是逐步移动的。该运动由向前和向后的步长组成,步长分别为2.8和-2.4 nm,几乎等于肌动蛋白亚基的长度(〜2.7 nm),与普遍接受的模型一致。但是,除了相当于单个肌动蛋白亚基长度的步骤外,还经常观察到相当于两个或三个亚基长度的步骤。我们的发现表明,mDia1步进和肌动蛋白聚合之间的偶联不是紧密而是松散的,这可以通过mDia1的多个结合状态来实现,从而为有效构建和调节纳米丝提供了生物分子的协同功能的见解。
更新日期:2018-09-25
中文翻译:

松散耦合肌动蛋白聚合的Formin mDia1的逐步纳米步骤。
Formin是肌动蛋白结合蛋白,可构成具有肌动蛋白丝长刺末端的纳米级机器,并充当肌动蛋白聚合和解聚的关键调节剂。为了维持肌动蛋白动力学的调节,已经提出了福明蛋白在单个肌动蛋白分子的每次缔合或解离处向新形成的带刺末端进行性移动。但是,建立运动机制的当前模型时没有直接观察到该运动的基本过程。在这里,使用光镊,我们证明了以纳米空间分辨率观察到的formin mDia1是逐步移动的。该运动由向前和向后的步长组成,步长分别为2.8和-2.4 nm,几乎等于肌动蛋白亚基的长度(〜2.7 nm),与普遍接受的模型一致。但是,除了相当于单个肌动蛋白亚基长度的步骤外,还经常观察到相当于两个或三个亚基长度的步骤。我们的发现表明,mDia1步进和肌动蛋白聚合之间的偶联不是紧密而是松散的,这可以通过mDia1的多个结合状态来实现,从而为有效构建和调节纳米丝提供了生物分子的协同功能的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号