当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Competition between two cysteines in covalent binding of biliverdin to phytochrome domains
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-25 , DOI: 10.1039/c8ob02262c Maria G. Khrenova 1, 2, 3, 4, 5 , Anna M. Kulakova 1, 2, 3, 4 , Alexander V. Nemukhin 1, 2, 3, 4, 6
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-25 , DOI: 10.1039/c8ob02262c Maria G. Khrenova 1, 2, 3, 4, 5 , Anna M. Kulakova 1, 2, 3, 4 , Alexander V. Nemukhin 1, 2, 3, 4, 6
Affiliation
|
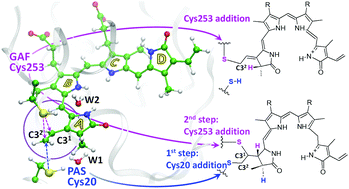
In this work, we disclose a mechanism of competing chemical reactions of protein assembly for a bacterial phytochrome using modern methods of molecular modeling. The recently designed variant of a near-infrared fluorescent protein miRFP670 shows novel and unexpected features of covalent binding of the biliverdin chromophore to cysteine residues in the phytochrome domains GAF and PAS. Upon protein assembly, biliverdin reacts either with a cysteine from GAF, or with two cysteines, one from GAF and one from PAS. We characterize computationally a model structure of the noncovalently bound biliverdin molecule inside the protein cleft of miRFP670 and model reactions of the covalent binding. Both cysteines, Cys20 (PAS) and Cys253 (GAF), are located close to the electrophile C32 atom of biliverdin and can act as nucleophiles. The nucleophilic attack of Cys253 from the GAF domain results in a single C–S bond formation with an activation energy of 16 kcal mol−1. Another pathway, leading to the biliverdin adduct with two C–S bonds, is characterized by lower energy barriers, less than 11 kcal mol−1. Competition between these reaction pathways explains the experimentally obtained mixture of both adducts. On the basis of our first simulations of covalent BV binding to the phytochrome domains, we propose an approach of a direct experimental validation of the reaction mechanisms using IR vibrational spectroscopy.
中文翻译:

Biliverdin与植物色素结构域的共价结合中的两个半胱氨酸之间的竞争
在这项工作中,我们公开了使用现代分子建模方法对细菌植物色素进行蛋白质装配竞争化学反应的机制。最近设计的近红外荧光蛋白miRFP670变体显示出biliverdin生色团与植物色素结构域GAF和PAS中的半胱氨酸残基共价结合的新颖且出乎意料的特征。蛋白质组装后,biliverdin与GAF的半胱氨酸或两种半胱氨酸反应,一种来自GAF,另一种来自PAS。我们在计算上表征了miRFP670蛋白裂隙内非共价结合的biliverdin分子的模型结构和共价结合的模型反应。半胱氨酸Cys20(PAS)和Cys253(GAF)均位于亲电试剂C3 2附近biliverdin的原子,可以充当亲核试剂。Cys253来自GAF域的亲核攻击导致单个C–S键形成,活化能为16 kcal mol -1。另一条途径导致带有两个C–S键的Biliverdin加合物,其特征在于较低的能垒,小于11 kcal mol -1。这些反应途径之间的竞争解释了实验获得的两种加合物的混合物。在我们对共价BV与植物色素结构域结合的第一个模拟的基础上,我们提出了使用红外振动光谱对反应机理进行直接实验验证的方法。
更新日期:2018-10-18
中文翻译:

Biliverdin与植物色素结构域的共价结合中的两个半胱氨酸之间的竞争
在这项工作中,我们公开了使用现代分子建模方法对细菌植物色素进行蛋白质装配竞争化学反应的机制。最近设计的近红外荧光蛋白miRFP670变体显示出biliverdin生色团与植物色素结构域GAF和PAS中的半胱氨酸残基共价结合的新颖且出乎意料的特征。蛋白质组装后,biliverdin与GAF的半胱氨酸或两种半胱氨酸反应,一种来自GAF,另一种来自PAS。我们在计算上表征了miRFP670蛋白裂隙内非共价结合的biliverdin分子的模型结构和共价结合的模型反应。半胱氨酸Cys20(PAS)和Cys253(GAF)均位于亲电试剂C3 2附近biliverdin的原子,可以充当亲核试剂。Cys253来自GAF域的亲核攻击导致单个C–S键形成,活化能为16 kcal mol -1。另一条途径导致带有两个C–S键的Biliverdin加合物,其特征在于较低的能垒,小于11 kcal mol -1。这些反应途径之间的竞争解释了实验获得的两种加合物的混合物。在我们对共价BV与植物色素结构域结合的第一个模拟的基础上,我们提出了使用红外振动光谱对反应机理进行直接实验验证的方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号