当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Base-mediated [2 + 4] cycloadditions of in situ formed azaoxyallyl cations with N-(2-chloromethyl)aryl amides
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-25 , DOI: 10.1039/c8ob02176g Qiaomei Jin 1, 2, 3, 4, 5 , Meng Gao 1, 2, 3, 4, 5 , Dongjian Zhang 1, 2, 3, 4, 5 , Cuihua Jiang 1, 2, 3, 4, 5 , Nan Yao 1, 2, 3, 4, 5 , Jian Zhang 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-25 , DOI: 10.1039/c8ob02176g Qiaomei Jin 1, 2, 3, 4, 5 , Meng Gao 1, 2, 3, 4, 5 , Dongjian Zhang 1, 2, 3, 4, 5 , Cuihua Jiang 1, 2, 3, 4, 5 , Nan Yao 1, 2, 3, 4, 5 , Jian Zhang 1, 2, 3, 4, 5
Affiliation
|
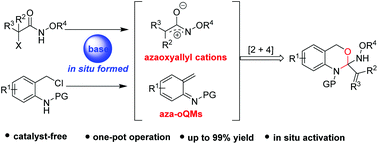
A base-mediated [2 + 4] annulation of in situ formed azaoxyallyl cations with in situ generated aza-oQMs has been realized. This one-pot cycloaddition process assembled the corresponding 1,4-dihydro-2H-benzo[d][1,3]oxazines in moderate to good yields (up to 99% yield).
中文翻译:

与N-(2-氯甲基)芳基酰胺进行的碱介导的[2 + 4]环加成反应原位形成的氮杂烯丙基阳离子
的碱介导的[2 + 4]环原位形成azaoxyallyl阳离子原位生成的氮杂ö QMS已经实现。此一锅环加成方法以中等至良好的产率(高达99%的产率)组装了相应的1,4-二氢-2 H-苯并[ d ] [1,3]恶嗪。
更新日期:2018-10-18
中文翻译:

与N-(2-氯甲基)芳基酰胺进行的碱介导的[2 + 4]环加成反应原位形成的氮杂烯丙基阳离子
的碱介导的[2 + 4]环原位形成azaoxyallyl阳离子原位生成的氮杂ö QMS已经实现。此一锅环加成方法以中等至良好的产率(高达99%的产率)组装了相应的1,4-二氢-2 H-苯并[ d ] [1,3]恶嗪。











































 京公网安备 11010802027423号
京公网安备 11010802027423号