当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
sp3 carbon–fluorine bond activation in 2,2-difluorohomoallylic alcohols via nucleophilic 5-endo-trig cyclisation: synthesis of 3-fluorinated furan derivatives†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-09-25 00:00:00 , DOI: 10.1039/c8cc04643c Takeshi Fujita 1, 2, 3, 4, 5 , Ryutaro Morioka 1, 2, 3, 4, 5 , Tomohiro Arita 1, 2, 3, 4, 5 , Junji Ichikawa 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2018-09-25 00:00:00 , DOI: 10.1039/c8cc04643c Takeshi Fujita 1, 2, 3, 4, 5 , Ryutaro Morioka 1, 2, 3, 4, 5 , Tomohiro Arita 1, 2, 3, 4, 5 , Junji Ichikawa 1, 2, 3, 4, 5
Affiliation
|
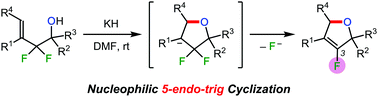
Nucleophilic 5-endo-trig cyclisation was achieved in 2,2-difluorohomoallylic alcohols. Upon treatment with potassium hydride, 2,2-difluorohomoallylic alcohols underwent an intramolecular SN2′-type reaction to afford 3-fluoro-2,5-dihydrofurans in high yields. In addition, the oxidation of these dihydrofurans formed 4-fluorofuran-2(5H)-ones. Thus, ring-fluorinated furan derivatives were efficiently obtained via allylic sp3 carbon–fluorine bond cleavage.
中文翻译:

通过亲核的5-内-trig环化作用使2,2-二氟同烯丙基醇中的 sp 3碳-氟键活化:3-氟代呋喃衍生物的合成†
在2,2-二氟均烯丙基醇中实现了亲核性5-内-trig环化。在用氢化钾处理,2,2- difluorohomoallylic醇进行分子内小号Ñ 2'-型反应,得到3-氟-2,5-二氢呋喃高收率。此外,这些二氢呋喃的氧化形成了4-氟呋喃-2(5 H)-one 。因此,通过烯丙基sp 3碳-氟键裂解可有效获得环氟化呋喃衍生物。
更新日期:2018-09-25
中文翻译:

通过亲核的5-内-trig环化作用使2,2-二氟同烯丙基醇中的 sp 3碳-氟键活化:3-氟代呋喃衍生物的合成†
在2,2-二氟均烯丙基醇中实现了亲核性5-内-trig环化。在用氢化钾处理,2,2- difluorohomoallylic醇进行分子内小号Ñ 2'-型反应,得到3-氟-2,5-二氢呋喃高收率。此外,这些二氢呋喃的氧化形成了4-氟呋喃-2(5 H)-one 。因此,通过烯丙基sp 3碳-氟键裂解可有效获得环氟化呋喃衍生物。










































 京公网安备 11010802027423号
京公网安备 11010802027423号