当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Synthesis of Mechanically Planar Chiral Rotaxanes
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-17 , DOI: 10.1002/anie.201808990 Michael A Jinks 1 , Alberto de Juan 1 , Mathieu Denis 1 , Catherine J Fletcher 1 , Marzia Galli 1 , Ellen M G Jamieson 1 , Florian Modicom 1 , Zhihui Zhang 1 , Stephen M Goldup 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-17 , DOI: 10.1002/anie.201808990 Michael A Jinks 1 , Alberto de Juan 1 , Mathieu Denis 1 , Catherine J Fletcher 1 , Marzia Galli 1 , Ellen M G Jamieson 1 , Florian Modicom 1 , Zhihui Zhang 1 , Stephen M Goldup 1
Affiliation
|
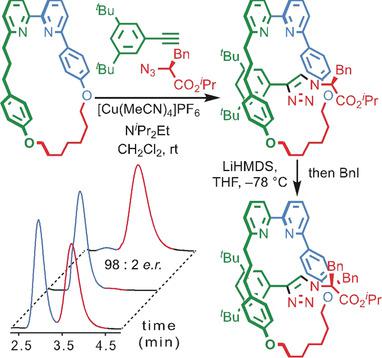
Chiral interlocked molecules in which the mechanical bond provides the sole stereogenic unit are typically produced with no control over the mechanical stereochemistry. Here we report a stereoselective approach to mechanically planar chiral rotaxanes in up to 98:2 d.r. using a readily available α‐amino acid‐derived azide. Symmetrization of the covalent stereocenter yields a rotaxane in which the mechanical bond provides the only stereogenic element.
中文翻译:

机械平面手性轮烷的立体选择性合成
机械键提供唯一立体单元的手性联锁分子通常是在不控制机械立体化学的情况下产生的。在这里,我们报告了一种机械平面手性轮烷的立体选择性方法,其比例高达 98:2 dr 。使用容易获得的α-氨基酸衍生的叠氮化物。共价立构中心的对称化产生轮烷,其中机械键提供唯一的立构元件。
更新日期:2018-10-17
中文翻译:

机械平面手性轮烷的立体选择性合成
机械键提供唯一立体单元的手性联锁分子通常是在不控制机械立体化学的情况下产生的。在这里,我们报告了一种机械平面手性轮烷的立体选择性方法,其比例高达 98:2 dr 。使用容易获得的α-氨基酸衍生的叠氮化物。共价立构中心的对称化产生轮烷,其中机械键提供唯一的立构元件。







































 京公网安备 11010802027423号
京公网安备 11010802027423号