当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bubble formation in catalyst pores; curse or blessing?†
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-09-24 00:00:00 , DOI: 10.1039/c8re00110c Roger Brunet Espinosa 1, 2, 3, 4, 5 , Michel H. G. Duits 2, 3, 4, 5, 6 , Daniel Wijnperlé 2, 3, 4, 5, 6 , Frieder Mugele 2, 3, 4, 5, 6 , Leon Lefferts 1, 2, 3, 4, 5
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-09-24 00:00:00 , DOI: 10.1039/c8re00110c Roger Brunet Espinosa 1, 2, 3, 4, 5 , Michel H. G. Duits 2, 3, 4, 5, 6 , Daniel Wijnperlé 2, 3, 4, 5, 6 , Frieder Mugele 2, 3, 4, 5, 6 , Leon Lefferts 1, 2, 3, 4, 5
Affiliation
|
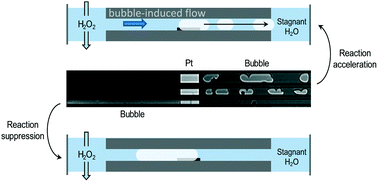
H2O2 decomposition experiments on Pt were performed in a glass microreactor, simulating arrays of catalyst pores. The formation of bubbles inside the model nanopores was observed with an optical microscope. It was found that the bubble initiation time strongly depends on the diffusion length and the H2O2 concentration. The amount of catalyst did not have a significant effect, suggesting that the reaction is diffusion limited. Results show that bubble formation can decrease the reaction rate by physically blocking the active sites, but also can accelerate the reaction by creating a forced convective flow inside the nanochannels due to bubble migration. Similar behaviour is likely to occur in a real catalyst and thus, a smart design of the catalytic support could be used to enhance reaction rates.
中文翻译:

在催化剂孔中形成气泡;诅咒还是祝福?†
在玻璃微反应器中对Pt进行H 2 O 2分解实验,模拟了催化剂孔的排列。用光学显微镜观察模型纳米孔内部的气泡的形成。发现气泡的起始时间强烈地取决于扩散长度和H 2 O 2。专注。催化剂的量没有显着影响,表明该反应受扩散限制。结果表明,气泡的形成可以通过物理阻断活性位点来降低反应速率,但是由于气泡的迁移,可以通过在纳米通道内部产生强制对流来加速反应。在实际的催化剂中可能会发生类似的行为,因此,可以使用催化载体的智能设计来提高反应速率。
更新日期:2018-09-24
中文翻译:

在催化剂孔中形成气泡;诅咒还是祝福?†
在玻璃微反应器中对Pt进行H 2 O 2分解实验,模拟了催化剂孔的排列。用光学显微镜观察模型纳米孔内部的气泡的形成。发现气泡的起始时间强烈地取决于扩散长度和H 2 O 2。专注。催化剂的量没有显着影响,表明该反应受扩散限制。结果表明,气泡的形成可以通过物理阻断活性位点来降低反应速率,但是由于气泡的迁移,可以通过在纳米通道内部产生强制对流来加速反应。在实际的催化剂中可能会发生类似的行为,因此,可以使用催化载体的智能设计来提高反应速率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号