当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of multisubstituted pyrroles by nickel-catalyzed arylative cyclizations of N-tosyl alkynamides†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-09-22 00:00:00 , DOI: 10.1039/c8cc06649c Simone M. Gillbard 1, 2, 3, 4, 5 , Chieh-Hsu Chung 1, 2, 3, 4, 5 , Somnath Narayan Karad 1, 2, 3, 4, 5 , Heena Panchal 1, 2, 3, 4, 5 , William Lewis 2, 5, 6, 7, 8 , Hon Wai Lam 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2018-09-22 00:00:00 , DOI: 10.1039/c8cc06649c Simone M. Gillbard 1, 2, 3, 4, 5 , Chieh-Hsu Chung 1, 2, 3, 4, 5 , Somnath Narayan Karad 1, 2, 3, 4, 5 , Heena Panchal 1, 2, 3, 4, 5 , William Lewis 2, 5, 6, 7, 8 , Hon Wai Lam 1, 2, 3, 4, 5
Affiliation
|
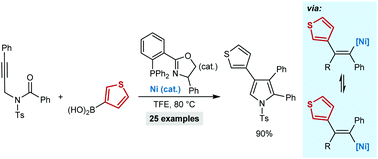
The synthesis of multisubstituted pyrroles by the nickel-catalyzed reaction of N-tosyl alkynamides with arylboronic acids is reported. These reactions are triggered by alkyne arylnickelation, followed by cyclization of the resulting alkenylnickel species onto the amide. The reversible E/Z isomerization of the alkenylnickel species is critical for cyclization. This method was applied to the synthesis of pyrroles that are precursors to BODIPY derivatives and a biologically active compound.
中文翻译:

由镍-催化的环化arylative吡咯多取代的合成Ñ -tosyl alkynamides †
报道了通过N-甲苯磺酰基炔基酰胺与芳基硼酸的镍催化反应合成多取代的吡咯。这些反应是由炔烃基芳基镍化反应触发,然后将所得的烯基镍基化合物环化到酰胺上。烯基镍物种的可逆E / Z异构化对于环化至关重要。该方法用于合成作为BODIPY衍生物和生物活性化合物的前体的吡咯。
更新日期:2018-09-22
中文翻译:

由镍-催化的环化arylative吡咯多取代的合成Ñ -tosyl alkynamides †
报道了通过N-甲苯磺酰基炔基酰胺与芳基硼酸的镍催化反应合成多取代的吡咯。这些反应是由炔烃基芳基镍化反应触发,然后将所得的烯基镍基化合物环化到酰胺上。烯基镍物种的可逆E / Z异构化对于环化至关重要。该方法用于合成作为BODIPY衍生物和生物活性化合物的前体的吡咯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号