当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Supplied Tumor Oxygenation through Separated Liposomal Delivery of H2O2 and Catalase for Enhanced Radio-Immunotherapy of Cancer
Nano Letters ( IF 9.6 ) Pub Date : 2018-09-24 00:00:00 , DOI: 10.1021/acs.nanolett.8b02720 Xuejiao Song 1, 2 , Jun Xu 2 , Chao Liang 2 , Yu Chao 2 , Qiutong Jin 2 , Chao Wang 2 , Meiwan Chen 1 , Zhuang Liu 2
Nano Letters ( IF 9.6 ) Pub Date : 2018-09-24 00:00:00 , DOI: 10.1021/acs.nanolett.8b02720 Xuejiao Song 1, 2 , Jun Xu 2 , Chao Liang 2 , Yu Chao 2 , Qiutong Jin 2 , Chao Wang 2 , Meiwan Chen 1 , Zhuang Liu 2
Affiliation
|
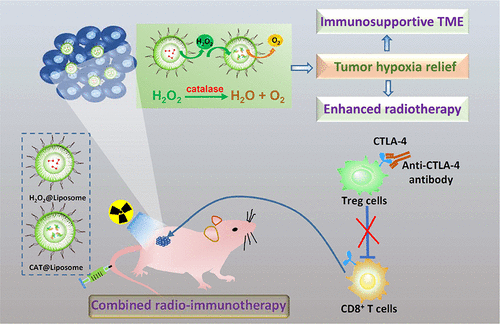
The recent years have witnessed the blooming of cancer immunotherapy, as well as their combinational use together with other existing cancer treatment techniques including radiotherapy. However, hypoxia is one of several causes of the immunosuppressive tumor microenvironment (TME). Herein, we develop an innovative strategy to relieve tumor hypoxia by delivering exogenous H2O2 into tumors and the subsequent catalase-triggered H2O2 decomposition. In our experiment, H2O2 and catalase are separately loaded within stealthy liposomes. After intravenous (iv) preinjection of [email protected], another dose of H2O2@liposome is injected 4 h later. The sustainably released H2O2 could be decomposed by [email protected], resulting in a long lasting effect in tumor oxygenation enhancement. As the result, the combination treatment by [email protected] plus H2O2@liposome offers remarkably enhanced therapeutic effects in cancer radiotherapy as observed in a mouse tumor model as well as a more clinically relevant patient-derived xenograft tumor model. Moreover, the relieved tumor hypoxia would reverse the immunosuppressive TME to favor antitumor immunities, further enhancing the combined radio-immunotherapy with cytotoxic T lymphocyte-associated antigen 4 (CTLA4) blockade. This work presents a simple yet effective strategy to promote tumor oxygenation via sequential delivering catalase and exogenous H2O2 into tumors using well-established liposomal carriers, showing great potential for clinical translation in radio-immunotherapy of cancer.
中文翻译:

通过单独的脂质体递送H 2 O 2和过氧化氢酶来自我补充肿瘤氧合,以增强癌症的放射免疫治疗。
近年来,见证了癌症免疫疗法的蓬勃发展,以及它们与包括放射疗法在内的其他现有癌症治疗技术的结合使用。但是,缺氧是免疫抑制肿瘤微环境(TME)的几种原因之一。本文中,我们开发了一种创新的策略,可通过将外源H 2 O 2输送到肿瘤中以及随后的过氧化氢酶触发的H 2 O 2分解来缓解肿瘤缺氧。在我们的实验中,H 2 O 2和过氧化氢酶分别装载在隐形脂质体中。静脉内(iv)预注射[电子邮件保护]后,再注射另一剂量的H 2 O 24小时后注射脂质体。可持续释放的H 2 O 2可以通过[电子邮件保护]分解,从而在增强肿瘤氧合作用方面具有长期持续的作用。结果,[电子邮件保护]加H 2 O 2的组合处理如在小鼠肿瘤模型以及更临床相关的患者来源的异种移植肿瘤模型中所观察到的,脂质体在癌症放射治疗中提供了显着增强的治疗效果。而且,缓解的肿瘤缺氧将逆转免疫抑制性TME从而有利于抗肿瘤免疫,进一步增强了联合放射免疫疗法与细胞毒性T淋巴细胞相关抗原4(CTLA4)的阻断作用。这项工作提出了一种简单而有效的策略,即通过使用成熟的脂质体载体将过氧化氢酶和外源H 2 O 2依次递送到肿瘤中来促进肿瘤氧合作用,显示出在癌症放射免疫疗法中进行临床翻译的巨大潜力。
更新日期:2018-09-24
中文翻译:

通过单独的脂质体递送H 2 O 2和过氧化氢酶来自我补充肿瘤氧合,以增强癌症的放射免疫治疗。
近年来,见证了癌症免疫疗法的蓬勃发展,以及它们与包括放射疗法在内的其他现有癌症治疗技术的结合使用。但是,缺氧是免疫抑制肿瘤微环境(TME)的几种原因之一。本文中,我们开发了一种创新的策略,可通过将外源H 2 O 2输送到肿瘤中以及随后的过氧化氢酶触发的H 2 O 2分解来缓解肿瘤缺氧。在我们的实验中,H 2 O 2和过氧化氢酶分别装载在隐形脂质体中。静脉内(iv)预注射[电子邮件保护]后,再注射另一剂量的H 2 O 24小时后注射脂质体。可持续释放的H 2 O 2可以通过[电子邮件保护]分解,从而在增强肿瘤氧合作用方面具有长期持续的作用。结果,[电子邮件保护]加H 2 O 2的组合处理如在小鼠肿瘤模型以及更临床相关的患者来源的异种移植肿瘤模型中所观察到的,脂质体在癌症放射治疗中提供了显着增强的治疗效果。而且,缓解的肿瘤缺氧将逆转免疫抑制性TME从而有利于抗肿瘤免疫,进一步增强了联合放射免疫疗法与细胞毒性T淋巴细胞相关抗原4(CTLA4)的阻断作用。这项工作提出了一种简单而有效的策略,即通过使用成熟的脂质体载体将过氧化氢酶和外源H 2 O 2依次递送到肿瘤中来促进肿瘤氧合作用,显示出在癌症放射免疫疗法中进行临床翻译的巨大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号