当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Fingerprints of Catalysis by Subsurface Hydrogen
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-09-24 00:00:00 , DOI: 10.1021/acscatal.8b02168 Irem Sen 1 , Andrew J. Gellman 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-09-24 00:00:00 , DOI: 10.1021/acscatal.8b02168 Irem Sen 1 , Andrew J. Gellman 1, 2
Affiliation
|
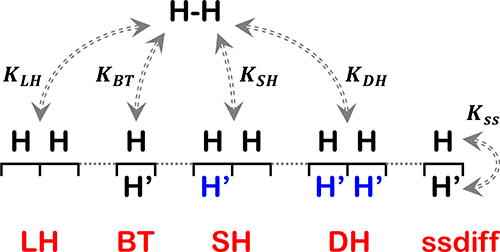
Mechanisms for catalytic H2–D2 exchange involving subsurface hydrogen, H′ (D′), have been analyzed to predict the ranges of reaction conditions over which the reaction orders, nH2 and nD2, can be used to distinguish among the various possible mechanisms. Four different mechanisms and combinations thereof have been considered: a Langmuir−Hinshelwood (LH) model, a breakthrough model invoking direct reaction between surface H(D) and subsurface H′ (D′), and two models invoking activation of H (D) by H′ (D′). In parallel, the kinetics of H2–D2 exchange have been measured over 90 AgxPd1–x alloy film samples with a continuous range of compositions: x = 0 → 1. For conditions with PH2 ≫ PD2, the reaction orders are found to be nH2 ≅ 0 and nD2 ≅ 1. The value of nH2 ≅ 0 is inconsistent with an LH mechanism under conditions with a hydrogen coverage of θ ≅ 1. A mechanism in which two subsurface H′ (D′) atoms promote recombinative desorption of H (D) atoms on the surface is consistent with the observed reaction orders under conditions in which the coverages on the surface and in the subsurface are θ ≅ 1 and θ′ ≈ 0, respectively.
中文翻译:

地下氢催化的动力学指纹图谱
分析了涉及地下氢H'(D')的催化H 2 -D 2交换机理,以预测反应条件的范围,在该范围内,反应顺序n H 2和n D 2可用于区分各种可能的机制。已经考虑了四种不同的机理及其组合:朗格缪尔-辛塞尔伍德(LH)模型,在表面H(D)和地下H'(D')之间进行直接反应的突破模型,以及在两个表面上激活H(D)的模型由H'(D')。同时,在90 Ag x Pd 1–上测量了H 2 –D 2交换的动力学。X具有连续范围的组合物的合金的膜样品: X = 0→1对于条件P ħ 2 » P d 2时,反应订单被发现是Ñ ħ 2 ≅0和Ñ d 2 ≅1的值ñ ħ 2 ≅0是与条件下的LH机构与θ的≅1.一种机构的氢覆盖,其中两个地下H'(d')的原子促进h的recombinative脱附(d)的表面上的原子是具有一致的不一致的在表面和亚表面的覆盖度分别为θ≅1和θ ′的条件下观察到的反应阶数 分别为≈0。
更新日期:2018-09-24
中文翻译:

地下氢催化的动力学指纹图谱
分析了涉及地下氢H'(D')的催化H 2 -D 2交换机理,以预测反应条件的范围,在该范围内,反应顺序n H 2和n D 2可用于区分各种可能的机制。已经考虑了四种不同的机理及其组合:朗格缪尔-辛塞尔伍德(LH)模型,在表面H(D)和地下H'(D')之间进行直接反应的突破模型,以及在两个表面上激活H(D)的模型由H'(D')。同时,在90 Ag x Pd 1–上测量了H 2 –D 2交换的动力学。X具有连续范围的组合物的合金的膜样品: X = 0→1对于条件P ħ 2 » P d 2时,反应订单被发现是Ñ ħ 2 ≅0和Ñ d 2 ≅1的值ñ ħ 2 ≅0是与条件下的LH机构与θ的≅1.一种机构的氢覆盖,其中两个地下H'(d')的原子促进h的recombinative脱附(d)的表面上的原子是具有一致的不一致的在表面和亚表面的覆盖度分别为θ≅1和θ ′的条件下观察到的反应阶数 分别为≈0。









































 京公网安备 11010802027423号
京公网安备 11010802027423号