当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding Dissolution and Crystallization with Imaging: A Surface Point of View.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-10-09 , DOI: 10.1021/acs.molpharmaceut.8b00840 Dunja Novakovic 1 , Antti Isomäki 2 , Bibi Pleunis 1 , Sara J Fraser-Miller 3 , Leena Peltonen 1 , Timo Laaksonen 4 , Clare J Strachan 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-10-09 , DOI: 10.1021/acs.molpharmaceut.8b00840 Dunja Novakovic 1 , Antti Isomäki 2 , Bibi Pleunis 1 , Sara J Fraser-Miller 3 , Leena Peltonen 1 , Timo Laaksonen 4 , Clare J Strachan 1
Affiliation
|
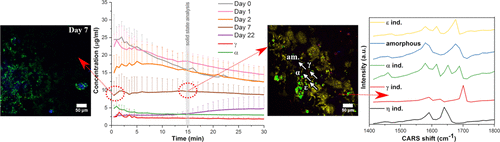
The tendency for crystallization during storage and administration is the most considerable hurdle for poorly water-soluble drugs formulated in the amorphous form. There is a need to better detect often subtle and complex surface crystallization phenomena and understand their influence on the critical quality attribute of dissolution. In this study, the interplay between surface crystallization of the amorphous form during storage and dissolution testing, and its influence on dissolution behavior, is analyzed for the first time with multimodal nonlinear optical imaging (coherent anti-Stokes Raman scattering (CARS) and sum frequency generation (SFG)). Complementary analyses are provided with scanning electron microscopy, X-ray diffraction and infrared and Raman spectroscopies. Amorphous indomethacin tablets were prepared and subjected to two different storage conditions (30 °C/23% RH and 30 °C/75% RH) for various durations and then dissolution testing using a channel flow-through device. Trace levels of surface crystallinity previously imaged with nonlinear optics after 1 or 2 days of storage did not significantly decrease dissolution and supersaturation compared to the freshly prepared amorphous tablets while more extensive crystallization after longer storage times did. Multimodal nonlinear optical imaging of the tablet surfaces after 15 min of dissolution revealed complex crystallization behavior that was affected by both storage condition and time, with up to four crystalline polymorphs simultaneously observed. In addition to the well-known α- and γ-forms, the less reported metastable ε- and η-forms were also observed, with the ε-form being widely observed in samples that had retained significant surface amorphousness during storage. This form was also prepared in the pure form and further characterized. Overall, this study demonstrates the potential value of nonlinear optical imaging, together with more established solid-state analysis methods, to understand complex surface crystallization behavior and its influence on drug dissolution during the development of amorphous drugs and dosage forms.
中文翻译:

通过成像了解溶解和结晶:表面观点。
对于以无定形形式配制的水溶性差的药物,在储存和给药过程中结晶的趋势是最大的障碍。需要更好地检测经常微妙和复杂的表面结晶现象,并了解它们对溶解的关键质量属性的影响。在这项研究中,首次使用多峰非线性光学成像(相干抗斯托克斯拉曼散射(CARS)和总频率)分析了非晶态晶体在储存和溶出度测试过程中表面结晶之间的相互作用及其对溶出行为的影响。 (SFG))。通过扫描电子显微镜,X射线衍射以及红外和拉曼光谱学进行补充分析。制备了吲哚美辛无定形片剂,并在两种不同的储存条件(30°C / 23%RH和30°C / 75%RH)下进行了不同的持续时间,然后使用通道流通设备进行了溶出度测试。与新鲜制备的无定形片剂相比,先前用非线性光学器件在储存1或2天后所成像的痕量表面结晶度不会显着降低溶解度和过饱和度,而在更长的储存时间后却能实现更广泛的结晶。溶解15分钟后,片剂表面的多峰非线性光学成像显示出复杂的结晶行为,该行为受储存条件和时间的影响,同时观察到多达四个晶体多晶型物。除了众所周知的α和γ形式,还观察到较少报道的亚稳态ε和η形式,其中ε形式广泛存在于在储存过程中保留了明显的表面非晶态的样品中。该形式也以纯形式制备并进一步表征。总的来说,这项研究证明了非线性光学成像的潜在价值,以及更成熟的固态分析方法,可以了解复杂的表面结晶行为及其对无定形药物和剂型开发过程中药物溶解的影响。
更新日期:2018-09-24
中文翻译:

通过成像了解溶解和结晶:表面观点。
对于以无定形形式配制的水溶性差的药物,在储存和给药过程中结晶的趋势是最大的障碍。需要更好地检测经常微妙和复杂的表面结晶现象,并了解它们对溶解的关键质量属性的影响。在这项研究中,首次使用多峰非线性光学成像(相干抗斯托克斯拉曼散射(CARS)和总频率)分析了非晶态晶体在储存和溶出度测试过程中表面结晶之间的相互作用及其对溶出行为的影响。 (SFG))。通过扫描电子显微镜,X射线衍射以及红外和拉曼光谱学进行补充分析。制备了吲哚美辛无定形片剂,并在两种不同的储存条件(30°C / 23%RH和30°C / 75%RH)下进行了不同的持续时间,然后使用通道流通设备进行了溶出度测试。与新鲜制备的无定形片剂相比,先前用非线性光学器件在储存1或2天后所成像的痕量表面结晶度不会显着降低溶解度和过饱和度,而在更长的储存时间后却能实现更广泛的结晶。溶解15分钟后,片剂表面的多峰非线性光学成像显示出复杂的结晶行为,该行为受储存条件和时间的影响,同时观察到多达四个晶体多晶型物。除了众所周知的α和γ形式,还观察到较少报道的亚稳态ε和η形式,其中ε形式广泛存在于在储存过程中保留了明显的表面非晶态的样品中。该形式也以纯形式制备并进一步表征。总的来说,这项研究证明了非线性光学成像的潜在价值,以及更成熟的固态分析方法,可以了解复杂的表面结晶行为及其对无定形药物和剂型开发过程中药物溶解的影响。









































 京公网安备 11010802027423号
京公网安备 11010802027423号