PLOS ONE ( IF 3.7 ) Pub Date : 2018-09-21 , DOI: 10.1371/journal.pone.0204283 Akanksha Agrawal , Richa Rathor , Ravi Kumar , Geetha Suryakumar , Lilly Ganju
|
Background
High altitude associated hypobaric hypoxia is one of the cellular and environmental perturbation that alters proteostasis network and push the healthy cell towards loss of muscle mass. The present study has elucidated the robust proteostasis network and signaling mechanism for skeletal muscle atrophy under chronic hypobaric hypoxia (CHH).
Methods
Male Sprague Dawley rats were exposed to simulated hypoxia equivalent to a pressure of 282 torr for different durations (1, 3, 7 and 14 days). After CHH exposure, skeletal muscle tissue was excised from the hind limb of rats for biochemical analysis.
Results
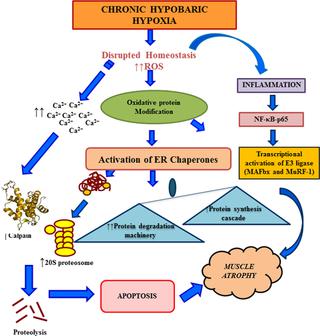
Chronic hypobaric hypoxia caused a substantial increase in protein oxidation and exhibited a greater activation of ER chaperones, glucose-regulated protein-78 (GRP-78) and protein disulphide isomerase (PDI) till 14d of CHH. Presence of oxidized proteins triggered the proteolytic systems, 20S proteasome and calpain pathway which were accompanied by a marked increase in [Ca2+]. Upregulated Akt pathway was observed upto 07d of CHH which was also linked with enhanced glycogen synthase kinase-3β (GSk-3β) expression, a negative regulator of Akt. Muscle-derived cytokines, tumor necrosis factor-α (TNF-α), interferon-ϒ (IFN-©) and interleukin-1β (IL-1β) levels significantly increased from 07d onwards. CHH exposure also upregulated the expression of nuclear factor kappa-B (NF-κB) and E3 ligase, muscle atrophy F-box-1 (Mafbx-1/Atrogin-1) and MuRF-1 (muscle ring finger-1) on 07d and 14d. Further, severe hypoxia also lead to increase expression of ER-associated degradation (ERAD) CHOP/ GADD153, Ub-proteasome and apoptosis pathway.
Conclusions
The disrupted proteostasis network was tightly coupled to degradative pathways, altered anabolic signaling, inflammation, and apoptosis under chronic hypoxia. Severe and prolonged hypoxia exposure affected the protein homeostasis which overwhelms the muscular system and tends towards skeletal muscle atrophy.
中文翻译:

改变蛋白质变形的网络在慢性低压缺氧引起的骨骼肌萎缩中的作用
背景
高海拔地区相关的低压缺氧是细胞和环境扰动之一,它会改变蛋白稳态网络并推动健康细胞向肌肉质量流失。本研究阐明了慢性低压缺氧(CHH)下骨骼肌萎缩的稳固的蛋白稳定网络和信号传导机制。
方法
将雄性Sprague Dawley大鼠在不同的时间段(1、3、7和14天)暴露于等效的低氧,相当于282托的压力。CHH暴露后,从大鼠后肢切除骨骼肌组织以进行生化分析。
结果
慢性低压缺氧导致蛋白质氧化显着增加,并在CHH的14天前表现出更大的ER分子伴侣,葡萄糖调节蛋白78(GRP-78)和蛋白二硫键异构酶(PDI)的活化。氧化蛋白的存在触发了蛋白水解系统,20S蛋白酶体和钙蛋白酶途径,并伴有[Ca 2+]。在CHH的第07天观察到Akt通路上调,这也与增强的糖原合酶激酶3β(GSk-3β)表达有关,后者是Akt的负调控子。肌肉来源的细胞因子,肿瘤坏死因子-α(TNF-α),干扰素-γ(IFN-©)和白介素-1β(IL-1β)的水平从07d开始显着增加。CHH暴露在07d时也上调了核因子kappa-B(NF-κB)和E3连接酶,肌肉萎缩F-box-1(Mafbx-1 / Atrogin-1)和MuRF-1(肌肉无名指1)的表达。和14d。此外,严重的缺氧还导致ER相关降解(ERAD)CHOP / GADD153,Ub-蛋白酶体和细胞凋亡途径的表达增加。
结论
在慢性低氧下,破坏的蛋白质稳定网络与降解途径紧密相关,改变了合成代谢的信号传导,炎症和细胞凋亡。长时间严重缺氧会影响蛋白质稳态,使肌肉系统不堪重负,并倾向于骨骼肌萎缩。



























 京公网安备 11010802027423号
京公网安备 11010802027423号