当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-based protein engineering enables prenyl donor switching of a fungal aromatic prenyltransferase
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-21 , DOI: 10.1039/c8ob02037j Peter Mai 1, 2, 3, 4 , Georg Zocher 4, 5, 6, 7 , Thilo Stehle 4, 5, 6, 7 , Shu-Ming Li 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-21 , DOI: 10.1039/c8ob02037j Peter Mai 1, 2, 3, 4 , Georg Zocher 4, 5, 6, 7 , Thilo Stehle 4, 5, 6, 7 , Shu-Ming Li 1, 2, 3, 4
Affiliation
|
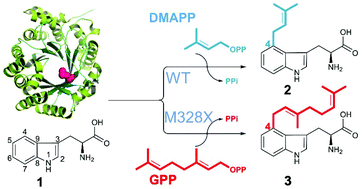
Microorganisms provide valuable enzyme machinery to assemble complex molecules. Fungal prenyltransferases (PTs) typically catalyse highly regiospecific prenylation reactions that are of significant pharmaceutical interest. While the majority of PTs accepts dimethylallyl diphosphate (DMAPP), very few such enzymes can use geranyl diphosphate (GPP) or farnesyl diphosphate (FPP) as donors. This catalytic gap prohibits the wide application of PTs for structural diversification. Structure-guided molecular modelling and site-directed mutagenesis of FgaPT2 from Aspergillus fumigatus led to the identification of the gatekeeping residue Met328 responsible for the prenyl selectivity and sets the basis for creation of GPP- and FPP-accepting enzymes. Site-saturation mutagenesis of the gatekeeping residue at position 328 in FgaPT2 revealed that the size of this side chain is the determining factor for prenyl selectivity, while its hydrophobicity is crucial for allowing DMAPP and GPP to bind.
中文翻译:

基于结构的蛋白质工程使真菌芳香性异戊二烯基转移酶的异戊二烯供体转换成为可能
微生物提供了有价值的酶机制来组装复杂的分子。真菌异戊二烯基转移酶(PTs)通常催化具有重要药物意义的高度区域特异性的异戊二烯化反应。虽然大多数PT都接受二磷酸二甲基烯丙酯(DMAPP),但很少有这类酶可以使用香叶基二磷酸(GPP)或法呢基二磷酸(FPP)作为供体。这种催化性的差距阻碍了PT在结构多样化方面的广泛应用。烟曲霉FgaPT2的结构指导分子建模和定点诱变导致鉴定出负责异戊二烯基选择性的守门残基Met328,并为创建GPP和FPP接受酶奠定了基础。FgaPT2中第328位守门残基的位点饱和诱变表明,该侧链的大小是异戊二烯基选择性的决定因素,而其疏水性对于允许DMAPP和GPP结合至关重要。
更新日期:2018-10-18
中文翻译:

基于结构的蛋白质工程使真菌芳香性异戊二烯基转移酶的异戊二烯供体转换成为可能
微生物提供了有价值的酶机制来组装复杂的分子。真菌异戊二烯基转移酶(PTs)通常催化具有重要药物意义的高度区域特异性的异戊二烯化反应。虽然大多数PT都接受二磷酸二甲基烯丙酯(DMAPP),但很少有这类酶可以使用香叶基二磷酸(GPP)或法呢基二磷酸(FPP)作为供体。这种催化性的差距阻碍了PT在结构多样化方面的广泛应用。烟曲霉FgaPT2的结构指导分子建模和定点诱变导致鉴定出负责异戊二烯基选择性的守门残基Met328,并为创建GPP和FPP接受酶奠定了基础。FgaPT2中第328位守门残基的位点饱和诱变表明,该侧链的大小是异戊二烯基选择性的决定因素,而其疏水性对于允许DMAPP和GPP结合至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号