当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Interfacial Reaction Trigged Propagation of Phase Transition from Surface into Bulk Lattice of Ni-Rich Layered Cathode
Chemistry of Materials ( IF 8.6 ) Pub Date : 2018-09-21 00:00:00 , DOI: 10.1021/acs.chemmater.8b01958 Lianfeng Zou 1 , Zhenyu Liu 2 , Wengao Zhao 3, 4 , Haiping Jia 3 , Jianming Zheng 3 , Yong Yang 4 , Guofeng Wang 2 , Ji-Guang Zhang 3 , Chongmin Wang 1
Chemistry of Materials ( IF 8.6 ) Pub Date : 2018-09-21 00:00:00 , DOI: 10.1021/acs.chemmater.8b01958 Lianfeng Zou 1 , Zhenyu Liu 2 , Wengao Zhao 3, 4 , Haiping Jia 3 , Jianming Zheng 3 , Yong Yang 4 , Guofeng Wang 2 , Ji-Guang Zhang 3 , Chongmin Wang 1
Affiliation
|
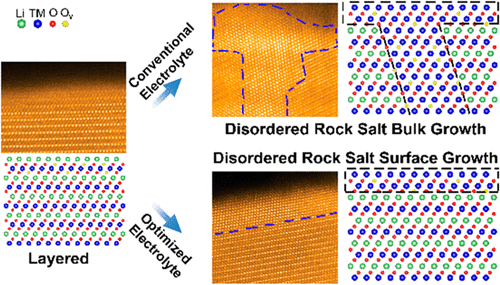
Solid–liquid interfacial reaction plays a crucial role for rechargeable batteries, and the reaction products are normally termed as solid liquid interface (SEI) layers. However, it remains far from clear as to how the solid–liquid interfacial reaction affects the bulk lattice. Here we demonstrate that the solid–liquid interfacial reaction extends beyond the SEI layer and essentially controls the bulk lattice behavior of the electrode. We discover that, for the Ni rich cathode (LiNi0.76Co0.10Mn0.14O2), adjusting the electrolyte chemistry triggers the anisotropic propagation of layered to rock salt phase transition into the bulk lattice, and consequently causes the progressive voltage and capacity decay. The ab initio computation suggests that, at the rock salt/layered interface, the interlayer migration of Ni ions is promoted, whereas the intralayer diffusion along the {003} channels is retarded. It is further suggested that the oxygen vacancies as a consequence of interfacial reactions preferentially diffuse along {104} planes, subsequently facilitating the rock salt bulk growth. The present observation provides a general view of how the solid–liquid interfacial reaction traverses beyond a geometrical interface and critically controls the underlying bulk lattice behavior and is of general importance for tailoring interfacial structure and chemistry for optimization of battery performance.
中文翻译:

固液界面反应触发了富镍层状阴极从表面到块状晶格相变的传播
固液界面反应对可充电电池起着至关重要的作用,反应产物通常称为固液界面(SEI)层。然而,关于固-液界面反应如何影响整体晶格,还远未弄清。在这里,我们证明了固液界面反应超出了SEI层,并从根本上控制了电极的整体晶格行为。我们发现,对于富镍阴极(LiNi 0.76 Co 0.10 Mn 0.14 O 2),调整电解质的化学性质会触发层状到岩盐相变向块状晶格的各向异性传播,并因此导致渐进的电压和容量衰减。从头算计算表明,在岩盐/层状界面处,Ni离子的层间迁移得到促进,而沿{003}通道的层内扩散受到阻碍。进一步表明,由于界面反应的结果,氧空位优先沿{104}面扩散,从而促进了盐盐块体的生长。
更新日期:2018-09-21
中文翻译:

固液界面反应触发了富镍层状阴极从表面到块状晶格相变的传播
固液界面反应对可充电电池起着至关重要的作用,反应产物通常称为固液界面(SEI)层。然而,关于固-液界面反应如何影响整体晶格,还远未弄清。在这里,我们证明了固液界面反应超出了SEI层,并从根本上控制了电极的整体晶格行为。我们发现,对于富镍阴极(LiNi 0.76 Co 0.10 Mn 0.14 O 2),调整电解质的化学性质会触发层状到岩盐相变向块状晶格的各向异性传播,并因此导致渐进的电压和容量衰减。从头算计算表明,在岩盐/层状界面处,Ni离子的层间迁移得到促进,而沿{003}通道的层内扩散受到阻碍。进一步表明,由于界面反应的结果,氧空位优先沿{104}面扩散,从而促进了盐盐块体的生长。



























 京公网安备 11010802027423号
京公网安备 11010802027423号