当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NIS-Mediated intermolecular hydroamination of allenamides with imidazole heterocycles: a facile protocol for the synthesis of allylic N,N-acetals†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-09-21 00:00:00 , DOI: 10.1039/c8nj03641a Yan Li 1, 2, 3, 4 , Guo Li Luo 1, 2, 3, 4 , Xiao Xiao Li 1, 2, 3, 4 , Zhi Gang Zhao 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-09-21 00:00:00 , DOI: 10.1039/c8nj03641a Yan Li 1, 2, 3, 4 , Guo Li Luo 1, 2, 3, 4 , Xiao Xiao Li 1, 2, 3, 4 , Zhi Gang Zhao 1, 2, 3, 4
Affiliation
|
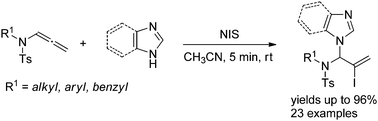
Allylic N,N-acetals are important intermediates in the synthesis of biologically active heterocycles and natural products. Herein, we report a facile protocol for the synthesis of this compound through N-iodosuccinimide-mediated hydroamination of allenamides by imidazole heterocycles. The reaction is regioselective, fast, and tolerant of a broad scope of imidazole and benzimidazole derivatives. The key intermediate is a conjugated sulfimide ion species that undergoes nucleophilic attack by imidazole to form the 1,2-adduct. Mixtures of N1- and N3-substituted isomers were obtained using asymmetrically substituted imidazoles. However, the 1,4-adduct was obtained using a tri-substituted imidazole. The efficiency of the gram-scale reaction suggests the potential industrial application of this synthetic method.
中文翻译:

NIS介导的烯丙基酰胺与咪唑杂环的分子间氢化胺化反应:合成烯丙基N,N-缩醛的简便方法†
烯丙基N,N-乙缩醛是生物活性杂环和天然产物合成中的重要中间体。在此,我们报道了通过咪唑杂环的N-碘琥珀酰亚胺介导的烯丙酰胺加氢胺化反应合成该化合物的简便方法。该反应是区域选择性的,快速的,并且耐受广泛范围的咪唑和苯并咪唑衍生物。关键中间体是共轭亚磺酰亚胺离子,其受到咪唑的亲核攻击而形成1,2-加合物。N 1-和N 3的混合物使用不对称取代的咪唑获得取代的异构体。然而,使用三取代的咪唑获得1,4-加合物。克级反应的效率表明该合成方法的潜在工业应用。
更新日期:2018-09-21
中文翻译:

NIS介导的烯丙基酰胺与咪唑杂环的分子间氢化胺化反应:合成烯丙基N,N-缩醛的简便方法†
烯丙基N,N-乙缩醛是生物活性杂环和天然产物合成中的重要中间体。在此,我们报道了通过咪唑杂环的N-碘琥珀酰亚胺介导的烯丙酰胺加氢胺化反应合成该化合物的简便方法。该反应是区域选择性的,快速的,并且耐受广泛范围的咪唑和苯并咪唑衍生物。关键中间体是共轭亚磺酰亚胺离子,其受到咪唑的亲核攻击而形成1,2-加合物。N 1-和N 3的混合物使用不对称取代的咪唑获得取代的异构体。然而,使用三取代的咪唑获得1,4-加合物。克级反应的效率表明该合成方法的潜在工业应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号