当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of Tertiary Alcohol via Chelation‐Assisted Nickel(II)‐Catalyzed Addition of Arylboronic Acids to Unactivated 1‐(Quinolin‐8‐yl)ethan‐1‐one
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-11 , DOI: 10.1002/adsc.201800943 Shutao Wu 1 , Weijie Guo 2 , Tao Wang 1 , Qingxiao Xie 1 , Jianhui Wang 1 , Guiyan Liu 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-11 , DOI: 10.1002/adsc.201800943 Shutao Wu 1 , Weijie Guo 2 , Tao Wang 1 , Qingxiao Xie 1 , Jianhui Wang 1 , Guiyan Liu 2
Affiliation
|
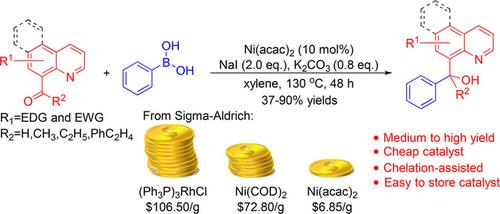
The synthesis of tertiary alcohol via chelation‐assisted nickel‐catalyzed addition of arylboronic acids to unactivated ketones was reported in this study. A series of substituted arylboronic acids was reacted with 1‐(quinolin‐8‐yl)ethan‐1‐one and its derivatives to provide various substituted 1‐aryl‐1‐(quinolin‐8‐yl)ethan‐1‐ols and relevant compounds in medium to good yields. The N‐directing group is essential for the addition reaction of arylboronic acids to these unactivated ketones. Nickel(II) acetylacetonate (10.0 mol%) in combination with sodium iodide (2 equiv.) and potassium carbonate (0.8 equiv.) was identified as the optimal catalytic system for the current transformations.
中文翻译:

通过螯合辅助镍(II)催化将芳基硼酸加成至未活化的1-(喹啉-8-基)乙烷-1-酮而形成叔醇
这项研究报道了通过螯合辅助镍催化将芳基硼酸加成到未活化的酮上来合成叔醇的方法。一系列取代的芳基硼酸与1-(喹啉-8-基)乙烷-1-酮及其衍生物反应生成各种取代的1-芳基-1-(喹啉-8-基)乙烷-1-醇及相关物质中等至高收率的化合物。N导向基团对于芳基硼酸与这些未活化的酮的加成反应至关重要。乙酰丙酮镍(II)(10.0 mol%)与碘化钠(2当量)和碳酸钾(0.8当量)的组合被确定为当前转化的最佳催化体系。
更新日期:2018-10-11
中文翻译:

通过螯合辅助镍(II)催化将芳基硼酸加成至未活化的1-(喹啉-8-基)乙烷-1-酮而形成叔醇
这项研究报道了通过螯合辅助镍催化将芳基硼酸加成到未活化的酮上来合成叔醇的方法。一系列取代的芳基硼酸与1-(喹啉-8-基)乙烷-1-酮及其衍生物反应生成各种取代的1-芳基-1-(喹啉-8-基)乙烷-1-醇及相关物质中等至高收率的化合物。N导向基团对于芳基硼酸与这些未活化的酮的加成反应至关重要。乙酰丙酮镍(II)(10.0 mol%)与碘化钠(2当量)和碳酸钾(0.8当量)的组合被确定为当前转化的最佳催化体系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号