当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biocatalytic Desymmetrization of Prochiral 3‐Aryl and 3‐Arylmethyl Glutaramides: Different Remote Substituent Effect on Catalytic Efficiency and Enantioselectivity
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-22 , DOI: 10.1002/adsc.201800956 Yu-Fei Ao 1 , Li-Bin Zhang 1 , Qi-Qiang Wang 1, 2 , De-Xian Wang 1, 2 , Mei-Xiang Wang 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-22 , DOI: 10.1002/adsc.201800956 Yu-Fei Ao 1 , Li-Bin Zhang 1 , Qi-Qiang Wang 1, 2 , De-Xian Wang 1, 2 , Mei-Xiang Wang 3
Affiliation
|
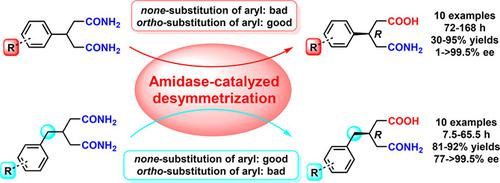
Catalyzed by an amidase‐containing Rhodococcus erythropolis AJ270 microbial whole cell catalyst in neutral phosphate buffer at 30 °C, desymmetric hydrolysis of a series of prochiral 3‐aryl and 3‐arylmethylglutaramides efficiently afforded 3‐substituted glutaric acid monoamides in up to 95% yield and >99.5% ee. Even far away from the reaction site, the substituents on the aryl still have a significant effect on the catalytic activity and enantioselectivity and different remote substituent effect was observed for the two types of substrates. The synthetic application of biocatalytic desymmetrization was demonstrated by the facile transformation of the obtained enantiopure (R)‐3‐substituted 4‐carbamoylbutanoic acid products to chiral dihydroquinolinone and δ‐lactone compounds.
中文翻译:

前手性3-芳基和3-芳基甲基戊二酰胺的生物催化脱对称:不同的远程取代基对催化效率和对映选择性的影响。
在30°C下于中性磷酸盐缓冲液中用含酰胺酶的红球菌红球菌AJ270微生物全细胞催化剂催化,一系列前手性3-芳基和3-芳基甲基戊二酰胺的不对称水解有效地提供了3-取代的戊二酸单酰胺,收率高达95%和> 99.5%ee。甚至远离反应位点,芳基上的取代基仍然对催化活性和对映选择性具有显着影响,并且对于两种类型的底物观察到不同的远程取代基效果。获得的对映纯(R)-3-取代的4-氨基甲酰基丁酸产物轻松转化为手性二氢喹啉酮和δ证明了生物催化脱对称的合成应用内酯化合物。
更新日期:2018-10-22
中文翻译:

前手性3-芳基和3-芳基甲基戊二酰胺的生物催化脱对称:不同的远程取代基对催化效率和对映选择性的影响。
在30°C下于中性磷酸盐缓冲液中用含酰胺酶的红球菌红球菌AJ270微生物全细胞催化剂催化,一系列前手性3-芳基和3-芳基甲基戊二酰胺的不对称水解有效地提供了3-取代的戊二酸单酰胺,收率高达95%和> 99.5%ee。甚至远离反应位点,芳基上的取代基仍然对催化活性和对映选择性具有显着影响,并且对于两种类型的底物观察到不同的远程取代基效果。获得的对映纯(R)-3-取代的4-氨基甲酰基丁酸产物轻松转化为手性二氢喹啉酮和δ证明了生物催化脱对称的合成应用内酯化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号