当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio‐ and Stereoselective Hydrosulfonylation of Electron‐Deficient Alkynes: Access to Both E‐ and Z‐β‐Sulfonyl‐α,β‐Unsaturated Carbonyl Compounds
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-16 , DOI: 10.1002/adsc.201801032 Wei Zhang 1 , Gabriel M. Johnson 2 , Zhi Guan 1 , Yan-Hong He 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-16 , DOI: 10.1002/adsc.201801032 Wei Zhang 1 , Gabriel M. Johnson 2 , Zhi Guan 1 , Yan-Hong He 1
Affiliation
|
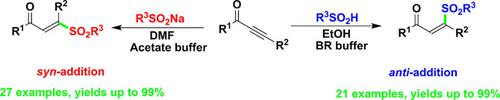
A metal‐free hydrosulfonylation of electron‐deficient alkynes with sodium sulfinates or sulfinic acids to access both E‐ and Z‐β‐sulfonyl‐α,β‐unsaturated carbonyl compounds has been developed. We propose that this reaction via a hydroxylallene intermediate delivers the thermodynamically stable E isomer, or via a concerted termolecular AdE3 mechanism affords Z isomer. The stereoselectivity of addition (syn or anti) can be controlled by varying the sulfonyl sources and acidic buffer solutions. This protocol exhibits broad substrate scope for internal or terminal alkynes including various substituted ynones and alkynyl esters. This approach is mild, efficient, operationally simple and easy to be scaled‐up.
中文翻译:

电子缺陷炔烃的区域和立体选择性加氢磺酰化:同时获得E-和Z-β-磺酰基-α,β-不饱和羰基化合物
已经开发出一种电子不足的炔烃,通过亚磺酸钠或亚磺酸进行无金属的氢磺酰化反应,以同时获得E-和Z - β-磺酰基-α,β-不饱和羰基化合物。我们建议该反应通过羟基丙二烯中间体传递热力学稳定的E异构体,或通过一致的分子Ad E 3机理提供Z异构体。加成的立体选择性(合成或反合成)可以通过改变磺酰基来源和酸性缓冲溶液来控制。该协议展示了内部或末端炔烃的广泛底物范围,包括各种取代的炔酮和炔基酯。这种方法温和,高效,操作简单且易于扩展。
更新日期:2018-10-16
中文翻译:

电子缺陷炔烃的区域和立体选择性加氢磺酰化:同时获得E-和Z-β-磺酰基-α,β-不饱和羰基化合物
已经开发出一种电子不足的炔烃,通过亚磺酸钠或亚磺酸进行无金属的氢磺酰化反应,以同时获得E-和Z - β-磺酰基-α,β-不饱和羰基化合物。我们建议该反应通过羟基丙二烯中间体传递热力学稳定的E异构体,或通过一致的分子Ad E 3机理提供Z异构体。加成的立体选择性(合成或反合成)可以通过改变磺酰基来源和酸性缓冲溶液来控制。该协议展示了内部或末端炔烃的广泛底物范围,包括各种取代的炔酮和炔基酯。这种方法温和,高效,操作简单且易于扩展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号