当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
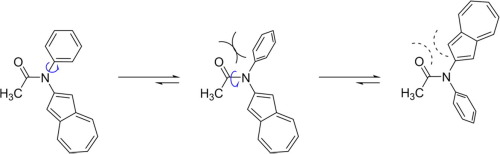
Conformational analysis of N-aryl-N-(2-azulenyl)acetamides
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2018-09-20 , DOI: 10.1016/j.tetlet.2018.09.053 Ai Ito , Takamasa Amaki , Ayako Ishii , Kazuo Fukuda , Ryu Yamasaki , Iwao Okamoto
中文翻译:

N-芳基-N-(2-氮烯基)乙酰胺的构象分析
更新日期:2018-09-20
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2018-09-20 , DOI: 10.1016/j.tetlet.2018.09.053 Ai Ito , Takamasa Amaki , Ayako Ishii , Kazuo Fukuda , Ryu Yamasaki , Iwao Okamoto
|
Aromatic amides bearing 2-azulenyl group on the amide nitrogen were synthesized and their structures were investigated. The π-electron density of the N-aryl group was found to influence the cis-trans conformational preferences of these compounds in solution. X-ray crystallography revealed that the plane of the 2-azulenyl ring has a strong tendency to lie coplanar with the amide plane when the azulene group is located on the same side as the amide oxygen atom.
中文翻译:

N-芳基-N-(2-氮烯基)乙酰胺的构象分析
合成了在酰胺氮上带有2-氮杂烯基的芳族酰胺,并对其结构进行了研究。发现N-芳基的π电子密度影响溶液中这些化合物的顺-反构象偏好。X射线晶体学表明,当氮杂基团与酰胺氧原子位于同一侧时,2-氮杂烯基环的平面具有很强的与酰胺平面共面的倾向。



























 京公网安备 11010802027423号
京公网安备 11010802027423号