当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Precise Enzymatic Cleavage Sites for Improved Bioactivity of siRNA Lipo-Polyplexes
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-09-21 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00585 Sören Reinhard 1 , Yanfang Wang 1 , Sebastian Dengler 1 , Ernst Wagner 1, 2
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-09-21 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00585 Sören Reinhard 1 , Yanfang Wang 1 , Sebastian Dengler 1 , Ernst Wagner 1, 2
Affiliation
|
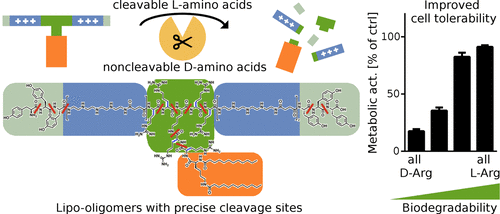
Sequence-defined cationic lipo-oligomers are potent siRNA carriers, forming stable lipo-polyplexes based on both electrostatic and hydrophobic interactions and, after endocytosis and endosomal protonation, facilitating the delivery of siRNA into the cytosol. After completion of the nucleic acid delivery process, carriers should be readily biodegradable to ensure minimum accumulation of amphiphilic molecules that are harmful to lysosomes and other intracellular organelles. Endolysosomal enzymes may degrade a surplus of carrier molecules left over in lysosomes and thereby facilitate the generation and rapid excretion of cleavage products. By solid-phase supported synthesis, a library of sequence-defined lipo-oligomers was generated containing artificial and natural amino acids comprising precise enzymatic cleavage sites. Incorporating either short cleavable l-arginine sequences (RR), noncleavable d-arginine linkers (rr), or varieties of both tailored the degradability of lipo-oligomers, as demonstrated upon incubation with the endolysosomal protease cathepsin B. Cleavage products were identified by MALDI-TOF mass spectrometry. The effect of improved intracellular degradation on cell tolerability was studied by transfecting Huh7-eGFPLuc and DU145-eGFPLuc cells. Positioning of enzymatic cleavage sites between a lipophilic diacyl domain and an ionizable oligocationic siRNA binding unit enabled efficient enzymatic degradation of the carrier and reduced the lytic potential under lysosomal conditions. Highly degradable carriers containing at least one l-arginine dipeptide linker significantly improved the viability of transfected cells without hampering gene silencing activity. Therefore, the precise integration of enzymatic cleavage sites in lipo-oligomers is a promising strategy toward biocompatible nucleic acid carriers.
中文翻译:

精确的酶切位点可提高siRNA脂质多聚体的生物活性
序列定义的阳离子脂质寡聚体是有效的siRNA载体,可基于静电和疏水相互作用形成稳定的脂质多聚体,在内吞作用和内体质子化后,有助于将siRNA传递到细胞质中。核酸递送过程完成后,载体应易于生物降解,以确保对溶酶体和其他细胞内细胞器有害的两亲分子的积累最少。溶酶体酶可以降解溶酶体中剩余的过量载体分子,从而促进裂解产物的产生和快速排泄。通过固相支持的合成,产生了包含人工和天然氨基酸的序列定义的脂质低聚物文库,所述人工和天然氨基酸包含精确的酶切位点。l-精氨酸序列(RR),不可切割的d-精氨酸接头(rr)或两者都针对脂寡聚体的降解性进行了定制,如与溶酶体蛋白酶组织蛋白酶B孵育时所证实的。裂解产物通过MALDI-TOF质谱法鉴定。通过转染Huh7-eGFPLuc和DU145-eGFPLuc细胞研究了改善的细胞内降解对细胞耐受性的影响。亲脂二酰基结构域和可电离的寡阳离子siRNA结合单元之间的酶切位点的位置使载体的有效酶降解,并降低了溶酶体条件下的裂解潜力。高度可降解的载体,至少包含1升-精氨酸二肽接头可显着提高转染细胞的活力,而不会影响基因沉默活性。因此,在脂寡聚体中酶促切割位点的精确整合是朝着生物相容性核酸载体发展的有前途的策略。
更新日期:2018-09-21
中文翻译:

精确的酶切位点可提高siRNA脂质多聚体的生物活性
序列定义的阳离子脂质寡聚体是有效的siRNA载体,可基于静电和疏水相互作用形成稳定的脂质多聚体,在内吞作用和内体质子化后,有助于将siRNA传递到细胞质中。核酸递送过程完成后,载体应易于生物降解,以确保对溶酶体和其他细胞内细胞器有害的两亲分子的积累最少。溶酶体酶可以降解溶酶体中剩余的过量载体分子,从而促进裂解产物的产生和快速排泄。通过固相支持的合成,产生了包含人工和天然氨基酸的序列定义的脂质低聚物文库,所述人工和天然氨基酸包含精确的酶切位点。l-精氨酸序列(RR),不可切割的d-精氨酸接头(rr)或两者都针对脂寡聚体的降解性进行了定制,如与溶酶体蛋白酶组织蛋白酶B孵育时所证实的。裂解产物通过MALDI-TOF质谱法鉴定。通过转染Huh7-eGFPLuc和DU145-eGFPLuc细胞研究了改善的细胞内降解对细胞耐受性的影响。亲脂二酰基结构域和可电离的寡阳离子siRNA结合单元之间的酶切位点的位置使载体的有效酶降解,并降低了溶酶体条件下的裂解潜力。高度可降解的载体,至少包含1升-精氨酸二肽接头可显着提高转染细胞的活力,而不会影响基因沉默活性。因此,在脂寡聚体中酶促切割位点的精确整合是朝着生物相容性核酸载体发展的有前途的策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号