当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental and Computational Studies Delineate the Role of Asparagine 177 in Hydride Transfer for E. coli Thymidylate Synthase
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1021/acscatal.8b02554 Ilya Gurevic 1 , Zahidul Islam 1 , Katarzyna Świderek 2 , Kai Trepka 1 , Ananda K. Ghosh 1 , Vicent Moliner 2 , Amnon Kohen 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1021/acscatal.8b02554 Ilya Gurevic 1 , Zahidul Islam 1 , Katarzyna Świderek 2 , Kai Trepka 1 , Ananda K. Ghosh 1 , Vicent Moliner 2 , Amnon Kohen 1
Affiliation
|
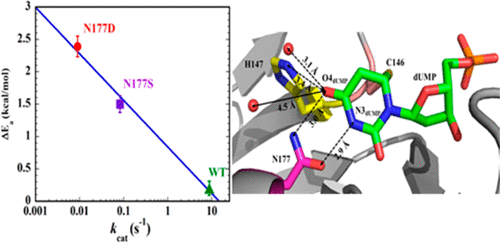
Thymidylate synthase (TSase), an enzyme responsible for the de novo biosynthesis of 2′-deoxythymidine 5′-monophosphate (thymidylate, dTMP) necessary for DNA synthesis, has been a drug target for decades. TSase is a highly conserved enzyme across species ranging from very primitive organisms to mammals. Among the many conserved active site residues, an asparagine (N177, using Escherichia coli residues numbering) appears to make direct hydrogen bonds with both the C4═O4 carbonyl of the 2′-deoxyuridine 5′-monophosphate (uridylate, dUMP) substrate and its pyrimidine ring’s N3. Recent studies have reassessed the TSase catalytic mechanism, focusing on the degree of negative charge accumulation at the O4 carbonyl of the substrate during two critical H-transfers–a proton abstraction and a hydride transfer. To obtain insights into the role of this conserved N177 on the hydride transfer, we examined its aspartic acid (D) and serine (S) mutants–each of which is expected to alter hydrogen bonding and charge stabilization around the C4═O4 carbonyl of the 2′-deoxyuridine 5′-monophosphate (uridylate, dUMP) substrate. Steady-state kinetics, substrate binding order studies and temperature-dependency analysis of intrinsic KIEs for the hydride transfer step of the TSase catalytic cycle suggest the active site of N177D is not precisely organized for that step. A smaller disruption was observed for N177S, which could be rationalized by partial compensation by water molecules and rearrangement of other residues toward preparation of the system for the hydride transfer under study. These experimental findings are qualitatively mirrored by QM/MM computational simulations, thereby shedding light on the sequence and synchronicity of steps in the TSase-catalyzed reaction. This information could potentially inform the design of mechanism-based drugs targeting this enzyme.
中文翻译:

实验和计算研究描述了天冬酰胺177在大肠杆菌胸苷酸合酶的氢化物转移中的作用
胸苷酸合酶(TSase)是负责DNA合成必需的2'-脱氧胸苷5'-单磷酸酯(thymidylate,dTMP)的从头生物合成的酶,几十年来一直是药物的靶标。TSase是一种非常保守的酶,适用于从非常原始的生物到哺乳动物的各种物种。在许多保守的活性位点残基中,使用大肠杆菌的天冬酰胺(N177残基编号)似乎与2'-脱氧尿苷5'-单磷酸酯(尿苷,dUMP)底物的C4 = O4羰基及其嘧啶环的N3都具有直接氢键。最近的研究重新评估了TSase的催化机理,重点研究了在两个关键的H转移(质子提取和氢化物转移)过程中底物O4羰基上负电荷积累的程度。为了深入了解这种保守的N177在氢化物转移中的作用,我们检查了其天冬氨酸(D)和丝氨酸(S)突变体-预期它们中的每一个都会改变氢键和C4═O4羰基周围的电荷稳定性。 2'-脱氧尿苷5'-单磷酸盐(尿苷,dUMP)底物。稳态动力学 对于TSase催化循环的氢化物转移步骤,底物结合顺序研究和内在KIE的温度依赖性分析表明N177D的活性位点在该步骤中不是精确组织的。观察到较小的N177S破坏,这可以通过水分子的部分补偿和其他残基的重排来合理化,以制备正在研究的氢化物转移系统。这些实验结果通过QM / MM计算仿真得到了定性的反映,从而使人们更加关注TSase催化反应中步骤的顺序和同步性。该信息可能潜在地指导针对该酶的基于机理的药物的设计。观察到较小的N177S破坏,这可以通过水分子的部分补偿和其他残基的重排来合理化,以制备正在研究的氢化物转移系统。这些实验结果通过QM / MM计算仿真得到了定性的反映,从而使人们更加关注TSase催化反应中步骤的顺序和同步性。该信息可能潜在地指导针对该酶的基于机理的药物的设计。观察到较小的N177S破坏,这可以通过水分子的部分补偿和其他残基的重排来合理化,以制备正在研究的氢化物转移系统。这些实验结果通过QM / MM计算仿真得到了定性的反映,从而使人们更加关注TSase催化反应中步骤的顺序和同步性。该信息可能潜在地指导针对该酶的基于机理的药物的设计。从而揭示了TSase催化反应中各步骤的顺序和同步性。该信息可能潜在地指导针对该酶的基于机理的药物的设计。从而揭示了TSase催化反应中各步骤的顺序和同步性。该信息可能潜在地指导针对该酶的基于机理的药物的设计。
更新日期:2018-09-20
中文翻译:

实验和计算研究描述了天冬酰胺177在大肠杆菌胸苷酸合酶的氢化物转移中的作用
胸苷酸合酶(TSase)是负责DNA合成必需的2'-脱氧胸苷5'-单磷酸酯(thymidylate,dTMP)的从头生物合成的酶,几十年来一直是药物的靶标。TSase是一种非常保守的酶,适用于从非常原始的生物到哺乳动物的各种物种。在许多保守的活性位点残基中,使用大肠杆菌的天冬酰胺(N177残基编号)似乎与2'-脱氧尿苷5'-单磷酸酯(尿苷,dUMP)底物的C4 = O4羰基及其嘧啶环的N3都具有直接氢键。最近的研究重新评估了TSase的催化机理,重点研究了在两个关键的H转移(质子提取和氢化物转移)过程中底物O4羰基上负电荷积累的程度。为了深入了解这种保守的N177在氢化物转移中的作用,我们检查了其天冬氨酸(D)和丝氨酸(S)突变体-预期它们中的每一个都会改变氢键和C4═O4羰基周围的电荷稳定性。 2'-脱氧尿苷5'-单磷酸盐(尿苷,dUMP)底物。稳态动力学 对于TSase催化循环的氢化物转移步骤,底物结合顺序研究和内在KIE的温度依赖性分析表明N177D的活性位点在该步骤中不是精确组织的。观察到较小的N177S破坏,这可以通过水分子的部分补偿和其他残基的重排来合理化,以制备正在研究的氢化物转移系统。这些实验结果通过QM / MM计算仿真得到了定性的反映,从而使人们更加关注TSase催化反应中步骤的顺序和同步性。该信息可能潜在地指导针对该酶的基于机理的药物的设计。观察到较小的N177S破坏,这可以通过水分子的部分补偿和其他残基的重排来合理化,以制备正在研究的氢化物转移系统。这些实验结果通过QM / MM计算仿真得到了定性的反映,从而使人们更加关注TSase催化反应中步骤的顺序和同步性。该信息可能潜在地指导针对该酶的基于机理的药物的设计。观察到较小的N177S破坏,这可以通过水分子的部分补偿和其他残基的重排来合理化,以制备正在研究的氢化物转移系统。这些实验结果通过QM / MM计算仿真得到了定性的反映,从而使人们更加关注TSase催化反应中步骤的顺序和同步性。该信息可能潜在地指导针对该酶的基于机理的药物的设计。从而揭示了TSase催化反应中各步骤的顺序和同步性。该信息可能潜在地指导针对该酶的基于机理的药物的设计。从而揭示了TSase催化反应中各步骤的顺序和同步性。该信息可能潜在地指导针对该酶的基于机理的药物的设计。



























 京公网安备 11010802027423号
京公网安备 11010802027423号