当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxopiperazine capping: Formation of oxopiperazine-containing peptoids via C-terminal cyclization
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-09-20 , DOI: 10.1016/j.tetlet.2018.09.047 Yunjee Lee , Jiwon Seo
中文翻译:

Oxopiperazine封顶:通过C末端环化形成含oxopiperazine的类肽
更新日期:2018-09-20
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-09-20 , DOI: 10.1016/j.tetlet.2018.09.047 Yunjee Lee , Jiwon Seo
|
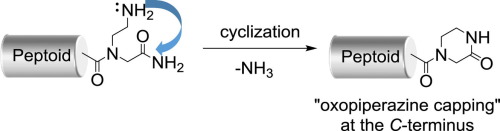
An unexpected side reaction was observed in peptoids containing a C-terminal carboxamide with a 2-aminoethyl side chain. The reaction proceeded via cyclization and release of NH3, resulting in C-terminal oxopiperazine formation, analogous to pyroglutamate formation from N-terminal glutamine in peptides. Reaction conditions that promote oxopiperazine formation were developed. In particular, the addition of organic bases accelerated the cyclization, thus providing a simple strategy for the post-synthetic C-terminal capping of peptoids.
中文翻译:

Oxopiperazine封顶:通过C末端环化形成含oxopiperazine的类肽
在含有带有2-氨基乙基侧链的C-末端羧酰胺的类肽中观察到了意外的副反应。该反应通过环化和NH 3的释放进行,导致C-末端氧代哌嗪的形成,类似于从肽中的N-末端谷氨酰胺形成焦谷氨酸。开发了促进氧哌哌嗪形成的反应条件。特别地,有机碱的添加加速了环化,从而为类肽的合成后的C-末端加帽提供了简单的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号