当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dirhodium(II)/P(t-Bu)3 catalyzed tandem reaction of α,β-unsaturated aldehydes with arylboronic acids
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-20 , DOI: 10.1039/c8ob01997e Ziling Ma 1, 2, 3, 4 , Yuanhua Wang 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-20 , DOI: 10.1039/c8ob01997e Ziling Ma 1, 2, 3, 4 , Yuanhua Wang 1, 2, 3, 4
Affiliation
|
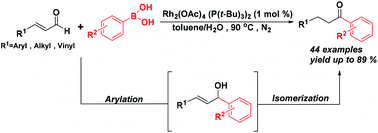
Phosphine ligated dirhodium(II) acetate is advocated as a catalyst for the synthesis of aryl alkyl ketones by the tandem reaction of α,β-unsaturated aromatic or aliphatic aldehydes with arylboronic acids. This tandem procedure included arylation followed by the isomerization reaction. This method exhibits good functional group tolerance and has a broad substrate scope. With the conjugated aldehydes, the one-step synthesis of γ,δ-unsaturated ketones was realized through this reaction. It is noteworthy that the length of the Rh–P bond is an important factor affecting catalytic reactions. The comparative analysis of the crystal structures of axially alkylphosphane and arylphosphane ligated dirhodium(II) acetate revealed that the shorter Rh–P bond length favors the isomerization process as compared to the longer one. In addition, the dirhodium(II) compound can be recovered after the completion of the reaction.
中文翻译:

Dirhodium(II)/ P(t -Bu)3催化α,β-不饱和醛与芳基硼酸的串联反应
提倡将膦连接的乙酸二铑(II)用作通过α,β-不饱和芳族或脂族醛与芳基硼酸的串联反应合成芳基烷基酮的催化剂。该串联过程包括芳基化,然后进行异构化反应。该方法表现出良好的官能团耐受性并且具有广泛的底物范围。通过共轭醛,通过该反应实现了γ,δ-不饱和酮的一步合成。值得注意的是,Rh-P键的长度是影响催化反应的重要因素。轴向烷基膦与芳基膦连接的二吡啶(II)晶体结构的比较分析)乙酸盐表明,与较长的Rh-P键相比,较短的Rh-P键长度有利于异构化过程。另外,反应完成后可以回收二吡啶(II)化合物。
更新日期:2018-10-18
中文翻译:

Dirhodium(II)/ P(t -Bu)3催化α,β-不饱和醛与芳基硼酸的串联反应
提倡将膦连接的乙酸二铑(II)用作通过α,β-不饱和芳族或脂族醛与芳基硼酸的串联反应合成芳基烷基酮的催化剂。该串联过程包括芳基化,然后进行异构化反应。该方法表现出良好的官能团耐受性并且具有广泛的底物范围。通过共轭醛,通过该反应实现了γ,δ-不饱和酮的一步合成。值得注意的是,Rh-P键的长度是影响催化反应的重要因素。轴向烷基膦与芳基膦连接的二吡啶(II)晶体结构的比较分析)乙酸盐表明,与较长的Rh-P键相比,较短的Rh-P键长度有利于异构化过程。另外,反应完成后可以回收二吡啶(II)化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号