当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvothermal liquefaction of alkali lignin to obtain a high yield of aromatic monomers while suppressing solvent consumption
Green Chemistry ( IF 9.3 ) Pub Date : 2018-09-20 , DOI: 10.1039/c8gc02460j Asim Riaz 1, 2, 3 , Deepak Verma 1, 2, 3 , Hassan Zeb 2, 3, 4, 5, 6 , Jeong Hyeon Lee 3, 7, 8, 9 , Jin Chul Kim 3, 7, 8, 9 , Sang Kyu Kwak 3, 7, 8, 9 , Jaehoon Kim 1, 2, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2018-09-20 , DOI: 10.1039/c8gc02460j Asim Riaz 1, 2, 3 , Deepak Verma 1, 2, 3 , Hassan Zeb 2, 3, 4, 5, 6 , Jeong Hyeon Lee 3, 7, 8, 9 , Jin Chul Kim 3, 7, 8, 9 , Sang Kyu Kwak 3, 7, 8, 9 , Jaehoon Kim 1, 2, 2, 3, 4
Affiliation
|
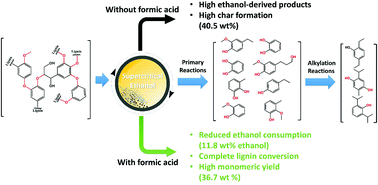
The unique physicochemical properties and high solubility of a wide range of biomass-derived feedstocks make sub- and supercritical alcohols promising media for thermochemical conversion to liquid fuels and value-added chemicals. Short-chain alcohols (C1–C3) not only hydrogenolyse a variety of recalcitrant feedstocks by donating in situ hydrogen, but also suppress the char formation by capping reactive intermediates. However, the beneficial features of supercritical alcohols also bring some demerits, such as their excessive decomposition and high consumption, which has been given cursory attention to date. Consequently, the aim of this study was to elucidate the role of sub- and supercritical alcohols as a hydrogen donor, their self-reactivity, their reactivity with the feedstock, the extent of their conversion under catalytic and non-catalytic conditions, and the detailed pathways to byproduct formation. Based on the solvent reactivity, the optimum conditions were investigated for the solvothermal liquefaction of recalcitrant alkali lignin to give a high yield of aromatic monomers with careful emphasis on the solvent consumption. The addition of formic acid instead of the more commonly used hydrodeoxygenation catalysts (e.g., CoMo/Al2O3, Ru/Al2O3) can not only suppress ethanol consumption significantly (from 42.3–46.8 wt% to 7 wt%), but can also result in complete lignin conversion by providing an excess amount of active hydrogen. The reaction at 350 °C for a short duration of 60 min led to the complete decomposition of alkali lignin and afforded a high yield of aromatic derivatives (36.7 wt%), while at the same time, suppressing ethanol consumption (11.8 wt%) and the formation of ethanol-derived liquid products. The alkylation of lignin-derived phenolic intermediates at the expense of the solvent is a time-dependent reaction, instead of the primary stabilization reaction. Molecular dynamics simulations using dilignol molecules revealed that the ethanol–formic acid mixture reduced the activation and thermal energies required for the dissociation of C–C and C–O bonds in the lignin structure.
中文翻译:

碱木质素的溶剂热液化在抑制溶剂消耗的同时获得高产率的芳族单体
多种生物质衍生原料的独特理化性质和高溶解度,使亚临界和超临界醇成为热化学转化为液体燃料和增值化学品的有前途的介质。短链醇(C 1 -C 3)不仅通过原位捐赠氢解了各种难降解的原料氢,但通过封端反应性中间体也抑制了焦炭的形成。然而,超临界醇的有益特征还带来一些缺点,例如它们的过度分解和高消耗,迄今为止已引起人们的粗略关注。因此,本研究的目的是阐明亚临界和超临界醇作为氢供体的作用,它们的自反应性,它们与原料的反应性,在催化和非催化条件下它们的转化程度以及详细的反应过程。副产物形成的途径。基于溶剂的反应性,研究了顽固性碱性木质素的溶剂热液化以得到高收率的芳族单体的最佳条件,并特别强调了溶剂的消耗。例如CoMo / Al 2 O 3,Ru / Al 2 O 3)不仅可以显着抑制乙醇消耗(从42.3–46.8 wt%到7 wt%),而且还可以通过提供过量的活性氢来实现木质素的完全转化。在350°C下短短60分钟的反应导致碱木质素完全分解,并提供了高产率的芳族衍生物(36.7 wt%),同时抑制了乙醇消耗(11.8 wt%)和形成乙醇衍生的液体产品。木质素衍生的酚类中间体的烷基化是以溶剂为代价的,是时间依赖性的反应,而不是一级稳定化反应。
更新日期:2018-10-30
中文翻译:

碱木质素的溶剂热液化在抑制溶剂消耗的同时获得高产率的芳族单体
多种生物质衍生原料的独特理化性质和高溶解度,使亚临界和超临界醇成为热化学转化为液体燃料和增值化学品的有前途的介质。短链醇(C 1 -C 3)不仅通过原位捐赠氢解了各种难降解的原料氢,但通过封端反应性中间体也抑制了焦炭的形成。然而,超临界醇的有益特征还带来一些缺点,例如它们的过度分解和高消耗,迄今为止已引起人们的粗略关注。因此,本研究的目的是阐明亚临界和超临界醇作为氢供体的作用,它们的自反应性,它们与原料的反应性,在催化和非催化条件下它们的转化程度以及详细的反应过程。副产物形成的途径。基于溶剂的反应性,研究了顽固性碱性木质素的溶剂热液化以得到高收率的芳族单体的最佳条件,并特别强调了溶剂的消耗。例如CoMo / Al 2 O 3,Ru / Al 2 O 3)不仅可以显着抑制乙醇消耗(从42.3–46.8 wt%到7 wt%),而且还可以通过提供过量的活性氢来实现木质素的完全转化。在350°C下短短60分钟的反应导致碱木质素完全分解,并提供了高产率的芳族衍生物(36.7 wt%),同时抑制了乙醇消耗(11.8 wt%)和形成乙醇衍生的液体产品。木质素衍生的酚类中间体的烷基化是以溶剂为代价的,是时间依赖性的反应,而不是一级稳定化反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号