当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In vitro evolution of an l-amino acid deaminase active on l-1-naphthylalanine†
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1039/c8cy01380b Roberta Melis 1, 2, 3, 4 , Elena Rosini 1, 2, 3, 4 , Valentina Pirillo 1, 2, 3, 4 , Loredano Pollegioni 1, 2, 3, 4 , Gianluca Molla 1, 2, 3, 4
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1039/c8cy01380b Roberta Melis 1, 2, 3, 4 , Elena Rosini 1, 2, 3, 4 , Valentina Pirillo 1, 2, 3, 4 , Loredano Pollegioni 1, 2, 3, 4 , Gianluca Molla 1, 2, 3, 4
Affiliation
|
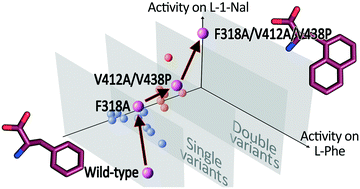
L-Amino acid deaminase from Proteus myxofaciens (PmaLAAD) is a promising biocatalyst for enantioselective biocatalysis that can be exploited to produce optically pure D-amino acids or α-keto acids. In this study, we improved the catalytic efficiency of PmaLAAD on L-1-naphthylalanine (L-1-Nal), a synthetic amino acid of biotechnological interest. Eight evolvable positions were identified by a molecular docking and evolutionary conservation analysis. These positions were subjected to site-saturation mutagenesis, producing “smaller but smarter” libraries of variants. The best variant (F318A/V412A/V438P PmaLAAD) possesses a ∼5-fold lower Km (0.17 mM) and a ∼7-fold higher catalytic efficiency (9.2 s−1 mM−1) on L-1-Nal than the wild-type enzyme. Molecular docking analysis suggests that the substitutions increase the active site volume, allowing better binding of the bulky L-1-Nal substrate. Bioconversion reactions showed that the F318A/V412A/V438P PmaLAAD variant outperforms the wild-type enzyme in the deracemization of D,L-1-Nal: the complete conversion of 0.6 mM of the L-enantiomer was achieved in about 15 min, which is ∼7.5-fold faster than the wild-type enzyme. In addition, the F318A/V412A/V438P PmaLAAD is efficiently employed, together with the M213G D-amino acid oxidase variant, to produce 1-naphtylpyruvate from racemic D,L-1-Nal in one pot.
中文翻译:

体外的演变升-氨基脱氨酶的活性上升-1-萘†
来自变形杆菌的L-氨基酸脱氨酶(PmaLAAD)是用于对映选择性生物催化的有前途的生物催化剂,其可用于产生光学纯的D-氨基酸或α-酮酸。在这项研究中,我们提高了PmaLAAD对L -1-萘基丙氨酸(L -1-Nal)的催化效率,L -1-萘丙氨酸是一种具有生物技术意义的合成氨基酸。通过分子对接和进化保守性分析确定了八个可进化的位置。这些位置经过位点饱和诱变,产生“更小但更聪明”的变体文库。最佳变体(F318A / V412A / V438P PmaLAAD)的K m低约5倍(0.17 mM)和对L -1-Nal的催化效率(9.2 s -1 mM -1)比野生型酶高约7倍。分子对接分析表明,取代增加了活性位点的体积,从而使更大的L -1-Nal底物更好地结合在一起。生物转化反应表明,F318A / V412A / V438P PmaLAAD变体在D,L -1-Nal的去消旋中优于野生型酶:在约15分钟内完成了0.6 mM的L-对映体的完全转化。比野生型酶快约7.5倍。此外,F318A / V412A / V438P PmaLAAD与M213G D一起有效使用-氨基酸氧化酶变体,在一个锅中从外消旋D,L -1-Nal生产1-萘丙酮酸。
更新日期:2018-09-20
中文翻译:

体外的演变升-氨基脱氨酶的活性上升-1-萘†
来自变形杆菌的L-氨基酸脱氨酶(PmaLAAD)是用于对映选择性生物催化的有前途的生物催化剂,其可用于产生光学纯的D-氨基酸或α-酮酸。在这项研究中,我们提高了PmaLAAD对L -1-萘基丙氨酸(L -1-Nal)的催化效率,L -1-萘丙氨酸是一种具有生物技术意义的合成氨基酸。通过分子对接和进化保守性分析确定了八个可进化的位置。这些位置经过位点饱和诱变,产生“更小但更聪明”的变体文库。最佳变体(F318A / V412A / V438P PmaLAAD)的K m低约5倍(0.17 mM)和对L -1-Nal的催化效率(9.2 s -1 mM -1)比野生型酶高约7倍。分子对接分析表明,取代增加了活性位点的体积,从而使更大的L -1-Nal底物更好地结合在一起。生物转化反应表明,F318A / V412A / V438P PmaLAAD变体在D,L -1-Nal的去消旋中优于野生型酶:在约15分钟内完成了0.6 mM的L-对映体的完全转化。比野生型酶快约7.5倍。此外,F318A / V412A / V438P PmaLAAD与M213G D一起有效使用-氨基酸氧化酶变体,在一个锅中从外消旋D,L -1-Nal生产1-萘丙酮酸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号