Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biological Evaluation and Docking Studies of Synthetic Oleanane-type Triterpenoids
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1021/acsomega.8b01034 Mariano Ortega-Muñoz , Fernando Rodríguez-Serrano 1 , Eduardo De los Reyes-Berbel , Nuria Mut-Salud , Fernando Hernández-Mateo , Andrea Rodríguez-López , José M. Garrido 1, 2 , F. Javier López-Jaramillo , Francisco Santoyo-González
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1021/acsomega.8b01034 Mariano Ortega-Muñoz , Fernando Rodríguez-Serrano 1 , Eduardo De los Reyes-Berbel , Nuria Mut-Salud , Fernando Hernández-Mateo , Andrea Rodríguez-López , José M. Garrido 1, 2 , F. Javier López-Jaramillo , Francisco Santoyo-González
Affiliation
|
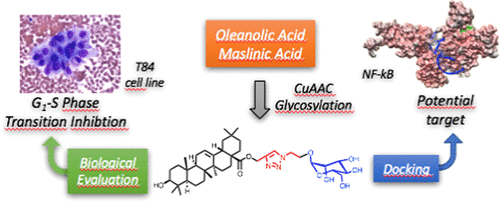
Saponins are potential wide-spectrum antitumor drugs, and copper(I) catalyzed azide–alkyne 1,3-dipolar cycloaddition is a suitable approach to synthesizing saponin-like compounds by regioselective glycosylation of the C2/C3 hydroxyl and C28 carboxylic groups of triterpene aglycones maslinic acid (MA) and oleanolic acid (OA). Biological studies on the T-84 human colon carcinoma cell line support the role of the hydroxyl groups at C2/C3, the influence of the aglycone, and the bulky nature of the substituents in C28. OA bearing a α-d-mannose moiety at C28 (compound 18) focused our interest because the estimated inhibitory concentration 50 was similar to that reported for ginsenoside Rh2 against colon cancer cells and it inhibits the G1–S phase transition affecting the cell viability and apoptosis. Considering that triterpenoids from natural sources have been identified as inhibitors of nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) signaling, docking studies were conducted to evaluate whether NF-κB may be a potential target. Results are consistent with the biological study and predict a similar binding mode of MA and compound 18 to the p52 subunit from NF-κB but not for OA. The fact that the binding site is shared by the NF-κB inhibitor 6,6-dimethyl-2-(phenylimino)-6,7-dihydrobenzo[d][1,3]oxathiol-4(5H)-one supports the result and points to NF-κB as a potential target of both MA and compound 18.
中文翻译:

合成齐墩果烷型三萜类化合物的生物学评价和对接研究
皂苷是潜在的广谱抗肿瘤药物,铜(I)催化的叠氮化物-炔烃1,3-偶极环加成是一种通过三萜糖苷配基的C2 / C3羟基和C28羧基区域选择性糖基化合成皂苷样化合物的合适方法。马来酸(MA)和齐墩果酸(OA)。T-84人结肠癌细胞系的生物学研究支持C2 / C3处羟基的作用,糖苷配基的影响以及C28中取代基的庞大性质。在C28(化合物18)带有一个α- d-甘露糖部分的OA引起了我们的兴趣,因为估计的抑制浓度50与报道的人参皂甙Rh2对结肠癌细胞的抑制浓度相似,并且它抑制了G 1-S相变影响细胞活力和细胞凋亡。考虑到来自天然来源的三萜类化合物已被确定为活化B细胞核因子κ-轻链增强子(NF-κB)信号的抑制剂,因此进行了对接研究以评估NF-κB是否可能是潜在的靶标。结果与生物学研究一致,并预测了MA和化合物18与NF-κB的p52亚基相似的结合模式,但对OA则没有。结合位点由NF-κB抑制剂6,6-二甲基-2-(苯基亚氨基)-6,7-二氢苯并[ d ] [1,3]草硫醇-4(5 H)-共享这一事实支持了结果并指出NF-κB是MA和化合物18的潜在靶标。
更新日期:2018-09-20
中文翻译:

合成齐墩果烷型三萜类化合物的生物学评价和对接研究
皂苷是潜在的广谱抗肿瘤药物,铜(I)催化的叠氮化物-炔烃1,3-偶极环加成是一种通过三萜糖苷配基的C2 / C3羟基和C28羧基区域选择性糖基化合成皂苷样化合物的合适方法。马来酸(MA)和齐墩果酸(OA)。T-84人结肠癌细胞系的生物学研究支持C2 / C3处羟基的作用,糖苷配基的影响以及C28中取代基的庞大性质。在C28(化合物18)带有一个α- d-甘露糖部分的OA引起了我们的兴趣,因为估计的抑制浓度50与报道的人参皂甙Rh2对结肠癌细胞的抑制浓度相似,并且它抑制了G 1-S相变影响细胞活力和细胞凋亡。考虑到来自天然来源的三萜类化合物已被确定为活化B细胞核因子κ-轻链增强子(NF-κB)信号的抑制剂,因此进行了对接研究以评估NF-κB是否可能是潜在的靶标。结果与生物学研究一致,并预测了MA和化合物18与NF-κB的p52亚基相似的结合模式,但对OA则没有。结合位点由NF-κB抑制剂6,6-二甲基-2-(苯基亚氨基)-6,7-二氢苯并[ d ] [1,3]草硫醇-4(5 H)-共享这一事实支持了结果并指出NF-κB是MA和化合物18的潜在靶标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号