Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Entry into 10b-aza-Analogues of Amaryllidaceae Alkaloids Reveals a Pronounced Electronic Effect on Antiviral Activity
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1021/acsomega.8b01987 Carla E. Brown 1 , Tiffany Kong 1 , James F. Britten 1 , Nick H. Werstiuk 1 , James McNulty 1 , Leonardo D’Aiuto 2 , Matthew Demers 2 , Vishwajit L. Nimgaonkar 2
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1021/acsomega.8b01987 Carla E. Brown 1 , Tiffany Kong 1 , James F. Britten 1 , Nick H. Werstiuk 1 , James McNulty 1 , Leonardo D’Aiuto 2 , Matthew Demers 2 , Vishwajit L. Nimgaonkar 2
Affiliation
|
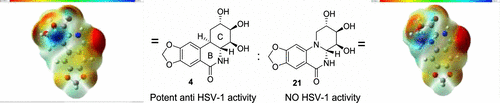
Development of a chiral pool-based synthesis of 10b-aza-analogues of biologically active Amaryllidaceae alkaloids is described, involving a concise reductive amination and condensation sequence, leading to ring-B/C-modified, fully functionalized ring-C derivatives. Differentiated anticancer and antiviral activities of these analogues are presented. Despite complete conformational and functional group overlap, the 10b-aza-analogues have diminished anticancer activity and no antiviral activity. These unprecedented electronic effects suggest a possible role for π-type secondary orbital interactions with the biological target.
中文翻译:

不对称进入10支芳烃科生物碱的b-氮杂类似物揭示了抗病毒活性的明显电子效应
描述了一种基于手性池的10种具有生物活性的芳科植物生物碱的b-氮杂类似物合成的合成方法,该方法涉及简明的还原胺化和缩合序列,从而产生环B / C修饰的,完全官能化的环C衍生物。介绍了这些类似物的差异化抗癌和抗病毒活性。尽管构象和功能基团完全重叠,但10 b-氮杂类似物的抗癌活性减弱,无抗病毒活性。这些空前的电子效应暗示了与生物靶标的π型次级轨道相互作用的可能作用。
更新日期:2018-09-20
中文翻译:

不对称进入10支芳烃科生物碱的b-氮杂类似物揭示了抗病毒活性的明显电子效应
描述了一种基于手性池的10种具有生物活性的芳科植物生物碱的b-氮杂类似物合成的合成方法,该方法涉及简明的还原胺化和缩合序列,从而产生环B / C修饰的,完全官能化的环C衍生物。介绍了这些类似物的差异化抗癌和抗病毒活性。尽管构象和功能基团完全重叠,但10 b-氮杂类似物的抗癌活性减弱,无抗病毒活性。这些空前的电子效应暗示了与生物靶标的π型次级轨道相互作用的可能作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号