当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of the UHPLC method for separation and characterization of major photolytic degradation products of trazodone by LC-MS and NMR†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1039/c8nj03545h Mohit Thummar 1, 2, 3, 4, 5 , Prinesh N. Patel 1, 2, 3, 4, 5 , Bhoopendra Singh Kushwah 1, 2, 3, 4, 5 , Gananadhamu Samanthula 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1039/c8nj03545h Mohit Thummar 1, 2, 3, 4, 5 , Prinesh N. Patel 1, 2, 3, 4, 5 , Bhoopendra Singh Kushwah 1, 2, 3, 4, 5 , Gananadhamu Samanthula 1, 2, 3, 4, 5
Affiliation
|
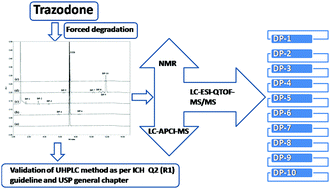
Drug degradation products are a type of drug impurity which affects the safety of the drug products. Trazodone (TZD) is an antidepressant drug subjected to forced degradation as per ICH embedded guidelines for predicting the drug degradation products. It undergoes degradation in acidic hydrolysis, peroxide induced oxidation and upon exposure to day light and results in the formation of ten degradation products (DPs). A UHPLC method was developed to separate TZD and its DPs using the stationary phase of an Acquity UPLC CSH C18 column (100 × 2.1 mm, 1.7 μm) and a mobile phase of solvent-A (10 mM ammonium acetate, pH 8.5) and solvent-B (methanol) at a flow rate of 0.25 mL min−1 in gradient elution. This method was transferred to a quadrupole time-of-flight tandem mass spectrometer (QTOF-MS/MS) for identification of the DPs. Very interestingly, four dimer DPs were found under photolytic degradation conditions and they are found to be isomers. The major isomer (DP-10) was isolated by using preparative HPLC and its structure was identified using proton and carbon NMR. Three N-oxide DPs were also observed and the site of N-oxidation was identified by using atmospheric pressure chemical ionization mass spectrometry (APCI-MS). The developed UHPLC method was validated as described in the ICH prescribed guidelines and USP general chapter on method validation. The proposed validated stability indicating assay method can be used for identification and quantification of TZD and its DPs in a quality control lab for product release testing and stability studies of the drug in much less time with greater selectivity.
中文翻译:

UHPLC方法在曲唑酮主要光解降解产物的LC-MS和NMR分离和表征中的应用†
药物降解产物是一种会影响药物安全性的药物杂质。曲唑酮(TZD)是一种抗抑郁药,按照ICH嵌入式指南中的预测药物降解产物的规定,会被迫降解。它在酸性水解,过氧化物诱导的氧化中以及在暴露于日光下会发生降解,并导致形成十种降解产物(DPs)。开发了UHPLC方法,使用Acquity UPLC CSH C 18色谱柱的固定相(100×2.1 mm,1.7μm)和溶剂-A的流动相(10 mM醋酸铵,pH 8.5)分离TZD及其DP。溶剂B(甲醇)的流速为0.25 mL min -1在梯度洗脱中。将该方法转移到四极杆飞行时间串联质谱仪(QTOF-MS / MS)上以鉴定DP。非常有趣的是,在光解降解条件下发现了四个二聚体DP,并且发现它们是异构体。使用制备型HPLC分离主要异构体(DP-10),并使用质子和碳NMR鉴定其结构。三N还观察到氧化DPs,并通过大气压化学电离质谱法(APCI-MS)鉴定了N-氧化的位点。如ICH规定的指南和USP关于方法验证的一般章节所述,对开发的UHPLC方法进行了验证。所提出的经过验证的稳定性指示测定方法可用于质量控制实验室中TZD及其DPs的鉴定和定量,以更少的时间和更大的选择性进行产品的释放测试和药物稳定性研究。
更新日期:2018-09-20
中文翻译:

UHPLC方法在曲唑酮主要光解降解产物的LC-MS和NMR分离和表征中的应用†
药物降解产物是一种会影响药物安全性的药物杂质。曲唑酮(TZD)是一种抗抑郁药,按照ICH嵌入式指南中的预测药物降解产物的规定,会被迫降解。它在酸性水解,过氧化物诱导的氧化中以及在暴露于日光下会发生降解,并导致形成十种降解产物(DPs)。开发了UHPLC方法,使用Acquity UPLC CSH C 18色谱柱的固定相(100×2.1 mm,1.7μm)和溶剂-A的流动相(10 mM醋酸铵,pH 8.5)分离TZD及其DP。溶剂B(甲醇)的流速为0.25 mL min -1在梯度洗脱中。将该方法转移到四极杆飞行时间串联质谱仪(QTOF-MS / MS)上以鉴定DP。非常有趣的是,在光解降解条件下发现了四个二聚体DP,并且发现它们是异构体。使用制备型HPLC分离主要异构体(DP-10),并使用质子和碳NMR鉴定其结构。三N还观察到氧化DPs,并通过大气压化学电离质谱法(APCI-MS)鉴定了N-氧化的位点。如ICH规定的指南和USP关于方法验证的一般章节所述,对开发的UHPLC方法进行了验证。所提出的经过验证的稳定性指示测定方法可用于质量控制实验室中TZD及其DPs的鉴定和定量,以更少的时间和更大的选择性进行产品的释放测试和药物稳定性研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号