Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
mRNA circularization by METTL3–eIF3h enhances translation and promotes oncogenesis
Nature ( IF 50.5 ) Pub Date : 2018-09-01 , DOI: 10.1038/s41586-018-0538-8 Junho Choe 1, 2 , Shuibin Lin 1, 2, 3 , Wencai Zhang 4 , Qi Liu 1, 2 , Longfei Wang 2 , Julia Ramirez-Moya 1, 5 , Peng Du 1, 2 , Wantae Kim 6, 7 , Shaojun Tang 8, 9 , Piotr Sliz 2 , Pilar Santisteban 5 , Rani E George 10, 11 , William G Richards 12 , Kwok-Kin Wong 13 , Nicolas Locker 14 , Frank J Slack 4, 15, 16 , Richard I Gregory 1, 2, 11, 15, 16
Nature ( IF 50.5 ) Pub Date : 2018-09-01 , DOI: 10.1038/s41586-018-0538-8 Junho Choe 1, 2 , Shuibin Lin 1, 2, 3 , Wencai Zhang 4 , Qi Liu 1, 2 , Longfei Wang 2 , Julia Ramirez-Moya 1, 5 , Peng Du 1, 2 , Wantae Kim 6, 7 , Shaojun Tang 8, 9 , Piotr Sliz 2 , Pilar Santisteban 5 , Rani E George 10, 11 , William G Richards 12 , Kwok-Kin Wong 13 , Nicolas Locker 14 , Frank J Slack 4, 15, 16 , Richard I Gregory 1, 2, 11, 15, 16
Affiliation
|
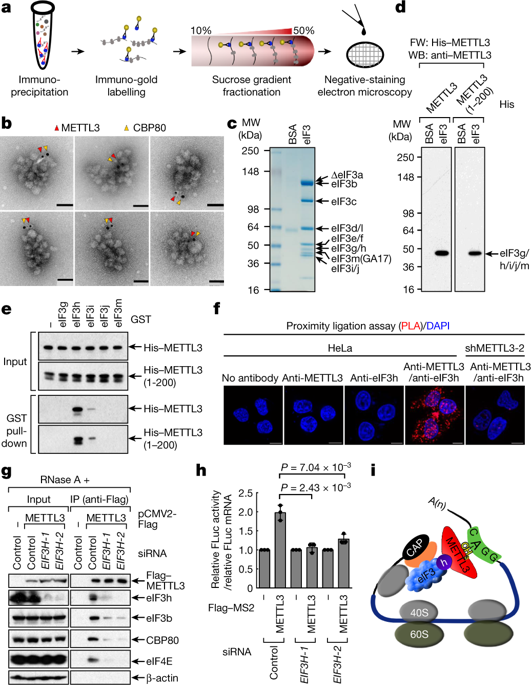
N6-methyladenosine (m6A) modification of mRNA is emerging as an important regulator of gene expression that affects different developmental and biological processes, and altered m6A homeostasis is linked to cancer1–5. m6A modification is catalysed by METTL3 and enriched in the 3′ untranslated region of a large subset of mRNAs at sites close to the stop codon5. METTL3 can promote translation but the mechanism and relevance of this process remain unknown1. Here we show that METTL3 enhances translation only when tethered to reporter mRNA at sites close to the stop codon, supporting a mechanism of mRNA looping for ribosome recycling and translational control. Electron microscopy reveals the topology of individual polyribosomes with single METTL3 foci in close proximity to 5′ cap-binding proteins. We identify a direct physical and functional interaction between METTL3 and the eukaryotic translation initiation factor 3 subunit h (eIF3h). METTL3 promotes translation of a large subset of oncogenic mRNAs—including bromodomain-containing protein 4—that is also m6A-modified in human primary lung tumours. The METTL3–eIF3h interaction is required for enhanced translation, formation of densely packed polyribosomes and oncogenic transformation. METTL3 depletion inhibits tumorigenicity and sensitizes lung cancer cells to BRD4 inhibition. These findings uncover a mechanism of translation control that is based on mRNA looping and identify METTL3–eIF3h as a potential therapeutic target for patients with cancer.METTL3, the enzyme responsible for m6A modification, influences translation by interacting with eIF3h to mediate looping between the regions near the stop codon and 5′ cap of mRNA.
中文翻译:

METTL3-eIF3h 的 mRNA 环化增强翻译并促进肿瘤发生
mRNA 的 N6-甲基腺苷 (m6A) 修饰正在成为影响不同发育和生物过程的基因表达的重要调节因子,并且改变的 m6A 稳态与癌症有关 1-5。m6A 修饰由 METTL3 催化,并在靠近终止密码子 5 的位点的一大类 mRNA 的 3' 非翻译区中富集。METTL3 可以促进翻译,但这一过程的机制和相关性仍然未知。在这里,我们表明 METTL3 仅在靠近终止密码子的位点与报告基因 mRNA 相连时才会增强翻译,支持 mRNA 循环用于核糖体循环和翻译控制的机制。电子显微镜揭示了在 5' 帽结合蛋白附近具有单个 METTL3 病灶的单个多核糖体的拓扑结构。我们确定了 METTL3 和真核翻译起始因子 3 亚基 h (eIF3h) 之间的直接物理和功能相互作用。METTL3 促进一大类致癌 mRNA 的翻译——包括含溴结构域的蛋白 4——在人类原发性肺肿瘤中也经过 m6A 修饰。METTL3-eIF3h 相互作用是增强翻译、形成密集的多核糖体和致癌转化所必需的。METTL3 耗竭抑制致瘤性并使肺癌细胞对 BRD4 抑制敏感。这些发现揭示了一种基于 mRNA 循环的翻译控制机制,并将 METTL3-eIF3h 鉴定为癌症患者的潜在治疗靶点。 METTL3,负责 m6A 修饰的酶,
更新日期:2018-09-01
中文翻译:

METTL3-eIF3h 的 mRNA 环化增强翻译并促进肿瘤发生
mRNA 的 N6-甲基腺苷 (m6A) 修饰正在成为影响不同发育和生物过程的基因表达的重要调节因子,并且改变的 m6A 稳态与癌症有关 1-5。m6A 修饰由 METTL3 催化,并在靠近终止密码子 5 的位点的一大类 mRNA 的 3' 非翻译区中富集。METTL3 可以促进翻译,但这一过程的机制和相关性仍然未知。在这里,我们表明 METTL3 仅在靠近终止密码子的位点与报告基因 mRNA 相连时才会增强翻译,支持 mRNA 循环用于核糖体循环和翻译控制的机制。电子显微镜揭示了在 5' 帽结合蛋白附近具有单个 METTL3 病灶的单个多核糖体的拓扑结构。我们确定了 METTL3 和真核翻译起始因子 3 亚基 h (eIF3h) 之间的直接物理和功能相互作用。METTL3 促进一大类致癌 mRNA 的翻译——包括含溴结构域的蛋白 4——在人类原发性肺肿瘤中也经过 m6A 修饰。METTL3-eIF3h 相互作用是增强翻译、形成密集的多核糖体和致癌转化所必需的。METTL3 耗竭抑制致瘤性并使肺癌细胞对 BRD4 抑制敏感。这些发现揭示了一种基于 mRNA 循环的翻译控制机制,并将 METTL3-eIF3h 鉴定为癌症患者的潜在治疗靶点。 METTL3,负责 m6A 修饰的酶,











































 京公网安备 11010802027423号
京公网安备 11010802027423号