Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermoresponsive Gel Embedded with Adipose Stem-Cell-Derived Extracellular Vesicles Promotes Esophageal Fistula Healing in a Thermo-Actuated Delivery Strategy
ACS Nano ( IF 15.8 ) Pub Date : 2018-09-19 00:00:00 , DOI: 10.1021/acsnano.8b00117 Amanda K. A. Silva 1 , Silvana Perretta 2, 3 , Guillaume Perrod 4 , Laetitia Pidial 4 , Véronique Lindner 5 , Florent Carn 1 , Shony Lemieux 1 , Damien Alloyeau 6 , Imane Boucenna 1 , Philippe Menasché 7 , Bernard Dallemagne 2 , Florence Gazeau 1 , Claire Wilhelm 1 , Christophe Cellier , Olivier Clément 4 , Gabriel Rahmi 4
ACS Nano ( IF 15.8 ) Pub Date : 2018-09-19 00:00:00 , DOI: 10.1021/acsnano.8b00117 Amanda K. A. Silva 1 , Silvana Perretta 2, 3 , Guillaume Perrod 4 , Laetitia Pidial 4 , Véronique Lindner 5 , Florent Carn 1 , Shony Lemieux 1 , Damien Alloyeau 6 , Imane Boucenna 1 , Philippe Menasché 7 , Bernard Dallemagne 2 , Florence Gazeau 1 , Claire Wilhelm 1 , Christophe Cellier , Olivier Clément 4 , Gabriel Rahmi 4
Affiliation
|
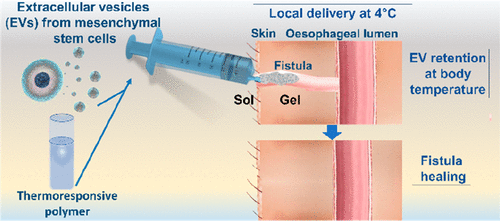
Extracellular vesicles (EVs) are increasingly envisioned as the next generation of biological pro-regenerative nanotherapeutic agents, as has already been demonstrated for heart, kidney, liver, and brain tissues; lung injury repair; and skin regeneration. Herein, we explore another potential EV therapeutic application, fistula healing, together with a local minimally invasive delivery strategy. Allogenic extracellular vesicles (EVs) from adipose tissue-derived stromal cells (ASCs) are administered in a porcine fistula model through a thermoresponsive Pluronic F-127 (PF-127) gel, injected locally at 4 °C and gelling at body temperature to retain EVs in the entire fistula tract. Complete fistula healing is reported to be 100% for the gel plus EVs group, 67% for the gel group, and 0% for the control, supporting the therapeutic use of Pluronic F-127 gel alone or combined with EVs. However, only the combination of gel and EVs results in a statistically significant (i) reduction of fibrosis, (ii) decline of inflammatory response, (iii) decrease in the density of myofibroblasts, and (iv) increase of angiogenesis. Overall, we demonstrate that ASC-EV delivery into a PF-127 gel represents a successful local minimally invasive strategy to induce a therapeutic effect in a swine fistula model. Our study presents prospects for EV administration strategies and for the management of post-operative fistulas.
中文翻译:

嵌入脂肪干细胞衍生的细胞外囊泡的热敏凝胶促进了热驱动递送策略中食管瘘的愈合。
细胞外囊泡(EVs)被越来越多地设想为下一代生物促再生纳米治疗剂,正如已经在心脏,肾脏,肝脏和脑组织中得到证实的那样。肺损伤修复;和皮肤再生。在本文中,我们探索了另一种潜在的EV治疗应用,即瘘管愈合以及局部微创递送策略。通过热响应性Pluronic F-127(PF-127)凝胶在猪瘘管模型中施用来自脂肪组织来源的基质细胞(ASC)的同种异体细胞外囊泡(EVs),在4°C局部注射并在体温下凝胶化以保留整个瘘管内的电动汽车。凝胶加EVs组的瘘管完全愈合据报道为100%,凝胶组为67%,对照组为0%,支持单独或与电动汽车结合使用Pluronic F-127凝胶的治疗用途。但是,只有凝胶和EV的组合才具有统计学上的显着性(i)纤维化减少,(ii)炎症反应下降,(iii)肌成纤维细胞密度降低和(iv)血管生成增加。总体而言,我们证明ASC-EV传递到PF-127凝胶代表了成功的局部微创策略,可在猪瘘管模型中诱导治疗效果。我们的研究提出了电动车管理策略和术后瘘管管理的前景。(iv)增加血管生成。总体而言,我们证明ASC-EV传递到PF-127凝胶代表了成功的局部微创策略,可在猪瘘管模型中诱导治疗效果。我们的研究提出了电动车管理策略和术后瘘管管理的前景。(iv)增加血管生成。总体而言,我们证明ASC-EV传递到PF-127凝胶代表了成功的局部微创策略,可在猪瘘管模型中诱导治疗效果。我们的研究提出了电动车管理策略和术后瘘管管理的前景。
更新日期:2018-09-19
中文翻译:

嵌入脂肪干细胞衍生的细胞外囊泡的热敏凝胶促进了热驱动递送策略中食管瘘的愈合。
细胞外囊泡(EVs)被越来越多地设想为下一代生物促再生纳米治疗剂,正如已经在心脏,肾脏,肝脏和脑组织中得到证实的那样。肺损伤修复;和皮肤再生。在本文中,我们探索了另一种潜在的EV治疗应用,即瘘管愈合以及局部微创递送策略。通过热响应性Pluronic F-127(PF-127)凝胶在猪瘘管模型中施用来自脂肪组织来源的基质细胞(ASC)的同种异体细胞外囊泡(EVs),在4°C局部注射并在体温下凝胶化以保留整个瘘管内的电动汽车。凝胶加EVs组的瘘管完全愈合据报道为100%,凝胶组为67%,对照组为0%,支持单独或与电动汽车结合使用Pluronic F-127凝胶的治疗用途。但是,只有凝胶和EV的组合才具有统计学上的显着性(i)纤维化减少,(ii)炎症反应下降,(iii)肌成纤维细胞密度降低和(iv)血管生成增加。总体而言,我们证明ASC-EV传递到PF-127凝胶代表了成功的局部微创策略,可在猪瘘管模型中诱导治疗效果。我们的研究提出了电动车管理策略和术后瘘管管理的前景。(iv)增加血管生成。总体而言,我们证明ASC-EV传递到PF-127凝胶代表了成功的局部微创策略,可在猪瘘管模型中诱导治疗效果。我们的研究提出了电动车管理策略和术后瘘管管理的前景。(iv)增加血管生成。总体而言,我们证明ASC-EV传递到PF-127凝胶代表了成功的局部微创策略,可在猪瘘管模型中诱导治疗效果。我们的研究提出了电动车管理策略和术后瘘管管理的前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号