当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N‐Heterocyclic Carbene‐Catalyzed in situ Activation of Alkynyl Acids for C−S Bond Formation: Access to Imidazo[2,1‐b][1,3]thiazinones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-12 , DOI: 10.1002/adsc.201800857 Kewen Sun 1 , Shiyi Jin 1 , Jindong Zhu 1 , Xinmiao Zhang 1 , Maoyu Gao 1 , Wanqi Zhang 1 , Tao Lu 1 , Ding Du 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-12 , DOI: 10.1002/adsc.201800857 Kewen Sun 1 , Shiyi Jin 1 , Jindong Zhu 1 , Xinmiao Zhang 1 , Maoyu Gao 1 , Wanqi Zhang 1 , Tao Lu 1 , Ding Du 1
Affiliation
|
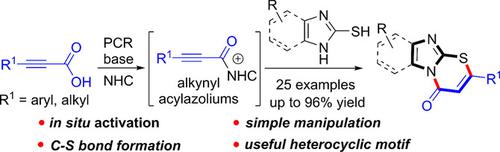
Alkynyl acids are first utilized as alkynyl acylazolium precursors through an in situ activation strategy for the efficient construction of carbon‐sulfur bond in a formal [3+3] annulation. This protocol offers a direct and rapid pathway for the synthesis of imidazo[2,1‐b][1,3]thiazinone skeleton, a useful heterocyclic class frequently found in many bioactive compounds. This reaction also has the advantages of scalability, readily available materials, and simple manipulation in open air.
中文翻译:

N-杂环碳烯催化炔酸活化形成C-S键:进入咪唑并[2,1-b] [1,3]噻嗪酮
炔酸首先通过原位活化策略用作炔基酰基la唑前体,以便在正式的[3 + 3]环空中有效构建碳硫键。该方案为合成咪唑并[2,1– b ] [1,3]噻嗪酮骨架提供了直接而快速的途径,该骨架是在许多生物活性化合物中经常发现的有用的杂环类。该反应还具有可扩展性,易于获得的材料以及在露天中易于操作的优点。
更新日期:2018-10-12
中文翻译:

N-杂环碳烯催化炔酸活化形成C-S键:进入咪唑并[2,1-b] [1,3]噻嗪酮
炔酸首先通过原位活化策略用作炔基酰基la唑前体,以便在正式的[3 + 3]环空中有效构建碳硫键。该方案为合成咪唑并[2,1– b ] [1,3]噻嗪酮骨架提供了直接而快速的途径,该骨架是在许多生物活性化合物中经常发现的有用的杂环类。该反应还具有可扩展性,易于获得的材料以及在露天中易于操作的优点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号