当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of enantiomerically-enriched N-aryl amino-amides via a Jocic-type reaction
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-09-19 , DOI: 10.1016/j.tetlet.2018.09.046 Christian Hobson , Michael S. Perryman , Gavin Kirby , Guy J. Clarkson , David J. Fox
中文翻译:

通过Jocic型反应合成对映体富集的N-芳基氨基酰胺
更新日期:2018-09-19
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-09-19 , DOI: 10.1016/j.tetlet.2018.09.046 Christian Hobson , Michael S. Perryman , Gavin Kirby , Guy J. Clarkson , David J. Fox
|
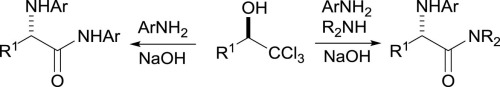
Enantiomerically-enriched secondary trichloromethyl-alcohols react with aryl amines to give enantiomerically-enriched α-N-arylamino-acid derivatives. The intermediate acid chlorides can react in situ with aryl or, regioselectively, with alkyl amines to give aryl or alkyl α-N-arylamino amides.
中文翻译:

通过Jocic型反应合成对映体富集的N-芳基氨基酰胺
对映体富集的仲三氯甲基醇与芳基胺反应,得到对映体富集的α - N-芳基氨基酸衍生物。中间体酰氯可与芳基原位反应,或与烷基胺区域选择性地反应,得到芳基或烷基α- N-芳基氨基酰胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号