当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
σ‐Aromaticity in a Fully Unsaturated Ring
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-10-31 , DOI: 10.1002/asia.201801279 Jingjing Wu 1 , Xin Liu 1 , Yulei Hao 1 , Hongjiang Chen 1 , Peifeng Su 1 , Wei Wu 1 , Jun Zhu 1
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-10-31 , DOI: 10.1002/asia.201801279 Jingjing Wu 1 , Xin Liu 1 , Yulei Hao 1 , Hongjiang Chen 1 , Peifeng Su 1 , Wei Wu 1 , Jun Zhu 1
Affiliation
|
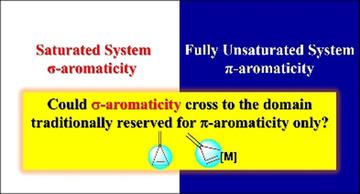
Aromaticity is one of the most fundamental and fascinating chemical topics, attracting both experimental and theoretical chemists owing to its many manifestations. Both σ‐ and π‐aromaticity can be classified depending on the character of the cyclic electron delocalization. In general, σ‐aromaticity stabilizes fully saturated rings with σ‐electron delocalization whereas the traditional π‐aromaticity describes the π‐conjugation in fully unsaturated rings. Here, we demonstrate a strong correlation between nucleus‐independent chemical shift (NICS) values and extra cyclic resonance energies (ECREs), which are used to evaluate the σ‐aromaticity in an unsaturated three‐membered ring (3MR) of cyclopropene, which were computed by molecular orbital (MO) theory and valence bond (VB) theory, respectively. Further study shows that the fully unsaturated ring in methylenecyclopropene and its metallic analogy is σ‐aromatic. Our findings revolutionize the fundamental knowledge of the concept of σ‐aromaticity, thus opening an avenue to design σ‐aromaticity in other fully unsaturated systems, which are traditionally reserved as the domain of π‐aromaticity.
中文翻译:

完全不饱和环中的σ-芳香性
芳香性是最基本,最引人入胜的化学主题之一,由于其许多表现形式而吸引了实验和理论化学家。可以根据循环电子离域的特征对σ和π芳香性进行分类。通常,σ-芳族化合物通过σ-电子离域来稳定完全饱和的环,而传统的π-芳族化合物描述了完全不饱和环中的π-共轭。在这里,我们证明了独立于核的化学位移(NICS)值与额外的循环共振能(ECREs)之间具有很强的相关性,这些值用于评估环丙烯的不饱和三元环(3MR)中的σ-芳族度,分别由分子轨道(MO)理论和价键(VB)理论计算。进一步的研究表明,亚甲基环丙烯中的完全不饱和环及其金属类似物是σ-芳族化合物。我们的发现彻底改变了人们对σ芳香性概念的基础知识,从而为设计其他传统上保留为π芳香性的完全不饱和体系中的σ芳香性开辟了道路。
更新日期:2018-10-31
中文翻译:

完全不饱和环中的σ-芳香性
芳香性是最基本,最引人入胜的化学主题之一,由于其许多表现形式而吸引了实验和理论化学家。可以根据循环电子离域的特征对σ和π芳香性进行分类。通常,σ-芳族化合物通过σ-电子离域来稳定完全饱和的环,而传统的π-芳族化合物描述了完全不饱和环中的π-共轭。在这里,我们证明了独立于核的化学位移(NICS)值与额外的循环共振能(ECREs)之间具有很强的相关性,这些值用于评估环丙烯的不饱和三元环(3MR)中的σ-芳族度,分别由分子轨道(MO)理论和价键(VB)理论计算。进一步的研究表明,亚甲基环丙烯中的完全不饱和环及其金属类似物是σ-芳族化合物。我们的发现彻底改变了人们对σ芳香性概念的基础知识,从而为设计其他传统上保留为π芳香性的完全不饱和体系中的σ芳香性开辟了道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号