当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoselective synthesis of isolated and fused fluorenones and their photophysical and antiviral properties
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-19 , DOI: 10.1039/c8ob01733f Ismail Althagafi 1, 2, 3, 4, 5 , Ranjay Shaw 1, 6, 7, 8 , Cheng-Run Tang 9, 10, 11, 12, 13 , Rahul Panwar 1, 6, 7, 8 , Shally Shally 1, 6, 7, 8 , Chanda Sinha 1, 6, 7, 8 , Amit Kumar 1, 14, 15, 16 , Yong-Tang Zheng 9, 10, 11, 12, 13 , Ramendra Pratap 1, 6, 7, 8
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-09-19 , DOI: 10.1039/c8ob01733f Ismail Althagafi 1, 2, 3, 4, 5 , Ranjay Shaw 1, 6, 7, 8 , Cheng-Run Tang 9, 10, 11, 12, 13 , Rahul Panwar 1, 6, 7, 8 , Shally Shally 1, 6, 7, 8 , Chanda Sinha 1, 6, 7, 8 , Amit Kumar 1, 14, 15, 16 , Yong-Tang Zheng 9, 10, 11, 12, 13 , Ramendra Pratap 1, 6, 7, 8
Affiliation
|
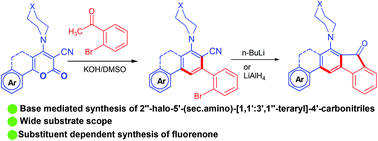
Highly functionalized fluorenones were synthesized by an intramolecular cyclization of 2′′-halo-[1,1′:3′,1′′-terphenyl]-4′-carbonitriles in the presence of n-butyllithium or lithium aluminium hydride. The precursor was synthesized by ring transformation of 2-oxo-6-aryl/heteroaryl-4-(sec.amino)-2H-pyran-3-carbonitriles or 2-oxobenzo[h]chromenes with o-bromo/chloro/fluoro-acetophenone under basic conditions in moderate yield. We performed the control experiment to understand the proposed mechanism and found that the presence of a secondary amine in the starting material directs the reactivity. The photophysical properties of 3-methoxy-7-(piperidin-1-yl)-5H-indeno[2,1-b]phenanthren-8(6H)-one was explored and solvent dependent emission was observed. These compounds were also tested against HIV-1 and low to moderate activity was observed.
中文翻译:

分离和融合的芴酮的化学选择性合成及其光物理和抗病毒特性
在正丁基锂或氢化铝锂的存在下,通过分子内环化2''-卤代-[1,1':3',1''-三联苯] -4'-腈来合成高度官能化的芴酮。通过将2-氧代-6-芳基/杂芳基-4-(仲氨基)-2 H-吡喃-3-甲腈或2-氧代苯并[ h ]色烯与邻-溴/氯/氟环转移来合成前体-苯乙酮在碱性条件下产率中等。我们进行了对照实验,以了解所提出的机理,并发现起始原料中仲胺的存在指导反应性。3-甲氧基-7-(哌啶-1-基)-5 H-茚三酮[2,1- b]的光物理性质探索了] phenanthren-8(6 H)-one并观察到溶剂依赖性发射。还测试了这些化合物的抗HIV-1活性,并观察到低至中等的活性。
更新日期:2018-10-18
中文翻译:

分离和融合的芴酮的化学选择性合成及其光物理和抗病毒特性
在正丁基锂或氢化铝锂的存在下,通过分子内环化2''-卤代-[1,1':3',1''-三联苯] -4'-腈来合成高度官能化的芴酮。通过将2-氧代-6-芳基/杂芳基-4-(仲氨基)-2 H-吡喃-3-甲腈或2-氧代苯并[ h ]色烯与邻-溴/氯/氟环转移来合成前体-苯乙酮在碱性条件下产率中等。我们进行了对照实验,以了解所提出的机理,并发现起始原料中仲胺的存在指导反应性。3-甲氧基-7-(哌啶-1-基)-5 H-茚三酮[2,1- b]的光物理性质探索了] phenanthren-8(6 H)-one并观察到溶剂依赖性发射。还测试了这些化合物的抗HIV-1活性,并观察到低至中等的活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号