Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydride Reduction of BaTiO3 − Oxyhydride Versus O Vacancy Formation
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-19 00:00:00 , DOI: 10.1021/acsomega.8b01368 Reji Nedumkandathil 1 , Aleksander Jaworski 1 , Jekabs Grins 1 , Diana Bernin 2 , Maths Karlsson 2 , Carin Eklöf-Österberg 2 , Alexandra Neagu 1 , Cheuk-Wai Tai 1 , Andrew J. Pell 1 , Ulrich Häussermann 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-19 00:00:00 , DOI: 10.1021/acsomega.8b01368 Reji Nedumkandathil 1 , Aleksander Jaworski 1 , Jekabs Grins 1 , Diana Bernin 2 , Maths Karlsson 2 , Carin Eklöf-Österberg 2 , Alexandra Neagu 1 , Cheuk-Wai Tai 1 , Andrew J. Pell 1 , Ulrich Häussermann 1
Affiliation
|
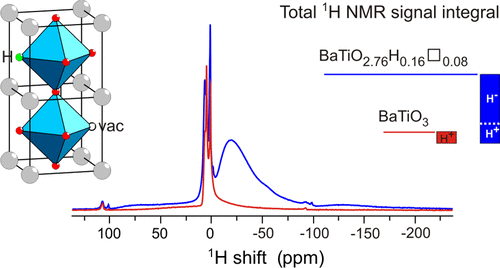
We investigated the hydride reduction of tetragonal BaTiO3 using the metal hydrides CaH2, NaH, MgH2, NaBH4, and NaAlH4. The reactions employed molar BaTiO3/H ratios of up to 1.8 and temperatures near 600 °C. The air-stable reduced products were characterized by powder X-ray diffraction (PXRD), transmission electron microscopy, thermogravimetric analysis (TGA), and 1H magic angle spinning (MAS) NMR spectroscopy. PXRD showed the formation of cubic products—indicative of the formation of BaTiO3–xHx—except for NaH. Lattice parameters were in a range between 4.005 Å (for NaBH4-reduced samples) and 4.033 Å (for MgH2-reduced samples). With increasing H/BaTiO3 ratio, CaH2-, NaAlH4-, and MgH2-reduced samples were afforded as two-phase mixtures. TGA in air flow showed significant weight increases of up to 3.5% for reduced BaTiO3, suggesting that metal hydride reduction yielded oxyhydrides BaTiO3–xHx with x values larger than 0.5. 1H MAS NMR spectroscopy, however, revealed rather low concentrations of H and thus a simultaneous presence of O vacancies in reduced BaTiO3. It has to be concluded that hydride reduction of BaTiO3 yields complex disordered materials BaTiO3–xHy□(x–y) with x up to 0.6 and y in a range 0.04–0.25, rather than homogeneous solid solutions BaTiO3–xHx. Resonances of (hydridic) H substituting O in the cubic perovskite structure appear in the −2 to −60 ppm spectral region. The large range of negative chemical shifts and breadth of the signals signifies metallic conductivity and structural disorder in BaTiO3–xHy□(x–y). Sintering of BaTiO3–xHy□(x–y) in a gaseous H2 atmosphere resulted in more ordered materials, as indicated by considerably sharper 1H resonances.
中文翻译:

BaTiO 3-氢氧化物与空位形成的氢化还原反应
我们研究了使用金属氢化物CaH 2,NaH,MgH 2,NaBH 4和NaAlH 4还原四方BaTiO 3的氢化物。该反应采用高达1.8的BaTiO 3 / H摩尔比和接近600℃的温度。空气稳定的还原产物通过粉末X射线衍射(PXRD),透射电子显微镜,热重分析(TGA)和1 H幻角旋转(MAS)NMR光谱进行表征。PXRD显示除了NaH以外,立方产物的形成(表明BaTiO 3– x H x的形成)。晶格参数范围为4.005Å(对于NaBH 4-还原样品)和4.033Å(对于MgH 2-还原样品)。随着H / BaTiO 3比的增加,提供了CaH 2-,NaAlH 4-和MgH 2-还原的样品,为两相混合物。对于还原的BaTiO 3,空气流中的TGA显示重量显着增加高达3.5%,这表明金属氢化物的还原产生了x值大于0.5的氢氧化物BaTiO 3– x H x。然而,1 H MAS NMR光谱显示还原的BaTiO 3中H的浓度相当低,因此同时存在O空位。必须得出的结论是,BaTiO 3的氢化物还原会生成复杂的无序材料BaTiO 3– x H y □ (x – y),x最高可达0.6,y的范围为0.04–0.25,而不是均匀的固溶体BaTiO 3– x ^ h X。在立方钙钛矿结构中用O取代(氢)H的共振出现在-2至-60 ppm的光谱范围内。负化学位移的大范围和信号的宽度表明BaTiO 3– x H y □ (x – y)。在气态H 2气氛中烧结BaTiO 3– x H y □(x – y)会产生更有序的材料,如1 H共振明显得多。
更新日期:2018-09-19
中文翻译:

BaTiO 3-氢氧化物与空位形成的氢化还原反应
我们研究了使用金属氢化物CaH 2,NaH,MgH 2,NaBH 4和NaAlH 4还原四方BaTiO 3的氢化物。该反应采用高达1.8的BaTiO 3 / H摩尔比和接近600℃的温度。空气稳定的还原产物通过粉末X射线衍射(PXRD),透射电子显微镜,热重分析(TGA)和1 H幻角旋转(MAS)NMR光谱进行表征。PXRD显示除了NaH以外,立方产物的形成(表明BaTiO 3– x H x的形成)。晶格参数范围为4.005Å(对于NaBH 4-还原样品)和4.033Å(对于MgH 2-还原样品)。随着H / BaTiO 3比的增加,提供了CaH 2-,NaAlH 4-和MgH 2-还原的样品,为两相混合物。对于还原的BaTiO 3,空气流中的TGA显示重量显着增加高达3.5%,这表明金属氢化物的还原产生了x值大于0.5的氢氧化物BaTiO 3– x H x。然而,1 H MAS NMR光谱显示还原的BaTiO 3中H的浓度相当低,因此同时存在O空位。必须得出的结论是,BaTiO 3的氢化物还原会生成复杂的无序材料BaTiO 3– x H y □ (x – y),x最高可达0.6,y的范围为0.04–0.25,而不是均匀的固溶体BaTiO 3– x ^ h X。在立方钙钛矿结构中用O取代(氢)H的共振出现在-2至-60 ppm的光谱范围内。负化学位移的大范围和信号的宽度表明BaTiO 3– x H y □ (x – y)。在气态H 2气氛中烧结BaTiO 3– x H y □(x – y)会产生更有序的材料,如1 H共振明显得多。











































 京公网安备 11010802027423号
京公网安备 11010802027423号