Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Key Role of the Local Hydrophobicity in the East Patch of Plastocyanins on Their Thermal Stability and Redox Properties
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-19 00:00:00 , DOI: 10.1021/acsomega.8b01612 José Luis Olloqui-Sariego 1 , Inmaculada Márquez 1 , Estrella Frutos-Beltrán 2 , Irene Díaz-Moreno 2 , Miguel A. De la Rosa 2 , Juan José Calvente 1 , Rafael Andreu 1 , Antonio Díaz-Quintana 2
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-19 00:00:00 , DOI: 10.1021/acsomega.8b01612 José Luis Olloqui-Sariego 1 , Inmaculada Márquez 1 , Estrella Frutos-Beltrán 2 , Irene Díaz-Moreno 2 , Miguel A. De la Rosa 2 , Juan José Calvente 1 , Rafael Andreu 1 , Antonio Díaz-Quintana 2
Affiliation
|
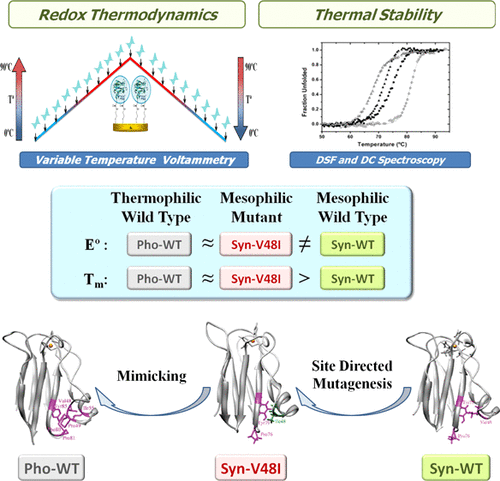
Understanding the molecular basis of the thermal stability and functionality of redox proteins has important practical applications. Here, we show a distinct thermal dependence of the spectroscopic and electrochemical properties of two plastocyanins from the thermophilic cyanobacterium Phormidium laminosum and their mesophilic counterpart from Synechocystis sp. PCC 6803, despite the similarity of their molecular structures. To explore the origin of these differences, we have mimicked the local hydrophobicity in the east patch of the thermophilic protein by replacing a valine of the mesophilic plastocyanin by isoleucine. Interestingly, the resulting mutant approaches the thermal stability, redox thermodynamics, and dynamic coupling of the flexible site motions of the thermophilic protein, indicating the existence of a close connection between the hydrophobic packing of the east patch region of plastocyanin and the functional control and stability of the oxidized and reduced forms of the protein.
中文翻译:

藻蓝蛋白东部斑块中局部疏水性对其热稳定性和氧化还原特性的关键作用
了解氧化还原蛋白的热稳定性和功能性的分子基础具有重要的实际应用。在这里,我们展示了光谱的不同的热依赖性和嗜热蓝藻2个plastocyanins的电化学性能纤细席laminosum并从他们的嗜温对应蓝藻sp。PCC 6803,尽管它们的分子结构相似。为了探索这些差异的起源,我们通过用异亮氨酸替代嗜温质体花色素的缬氨酸来模拟嗜热蛋白东面的局部疏水性。有趣的是,产生的突变体接近热稳定性,热还原蛋白的热力学和嗜热蛋白的柔性位点运动的动态耦合,表明质体花青素的东部斑片区域的疏水堆积与功能控制和稳定性之间存在紧密的联系。蛋白质的氧化和还原形式。
更新日期:2018-09-19
中文翻译:

藻蓝蛋白东部斑块中局部疏水性对其热稳定性和氧化还原特性的关键作用
了解氧化还原蛋白的热稳定性和功能性的分子基础具有重要的实际应用。在这里,我们展示了光谱的不同的热依赖性和嗜热蓝藻2个plastocyanins的电化学性能纤细席laminosum并从他们的嗜温对应蓝藻sp。PCC 6803,尽管它们的分子结构相似。为了探索这些差异的起源,我们通过用异亮氨酸替代嗜温质体花色素的缬氨酸来模拟嗜热蛋白东面的局部疏水性。有趣的是,产生的突变体接近热稳定性,热还原蛋白的热力学和嗜热蛋白的柔性位点运动的动态耦合,表明质体花青素的东部斑片区域的疏水堆积与功能控制和稳定性之间存在紧密的联系。蛋白质的氧化和还原形式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号