当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glucose Glycation of α-Lactalbumin and β-Lactoglobulin in Glycerol Solutions
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-09-19 00:00:00 , DOI: 10.1021/acs.jafc.8b03544 Xiaoxia Chen 1, 2 , Lina Zhang 1, 2 , Bhesh Bhandari 3 , Peng Zhou 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-09-19 00:00:00 , DOI: 10.1021/acs.jafc.8b03544 Xiaoxia Chen 1, 2 , Lina Zhang 1, 2 , Bhesh Bhandari 3 , Peng Zhou 1, 2
Affiliation
|
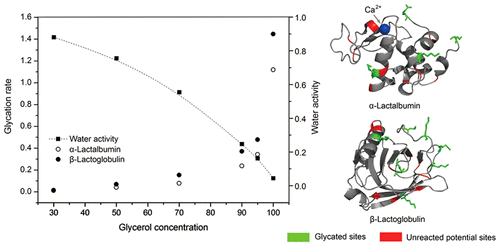
The glucose glycation of α-lactalbumin and β-lactoglobulin at 50 °C in a glycerol-based liquid system was investigated to evaluate the effect of water activity on glycation and site-specificity in a glycerol matrix. Glycation extent during the reaction was determined using the o-phthalaldehyde (OPA) method as well as ultraperformance liquid chromatography combined with electro-spray ionization mass spectrum (UPLC-ESI-MS) analysis. Glycation sites were identified by data-independent acquisition LC–MS (LC-MSE). The surface potential achieved by PyMOL and the tertiary structure determined by circular dichroism (CD) were used to assist the analysis of the glycation site-specificity in the glycerol matrix. The water activity of glycerol solutions was negatively correlated to the glycerol concentration. Results showed that the initial glycation rate in glycerol matrix was fitted to a linear equation in the first 48 h. Glycation accelerated with the increase of glycerol concentration, namely, the decrease of water activity, regardless of the native structure of the protein. The glycation sites were identical at a similar DSP although achieved at different water activities, with 4 and 7 sites detected in α-lactalbumin and β-lactoglobulin, respectively. However, compared with the glycation sites in a water-based matrix, the site-specificity of glycation was affected by the glycerol matrix, depending on the native structure of the proteins. Glycation was prone to occur at the reactive sites distributed on the surface of the proteins, particularly in the region with positive potential.
中文翻译:

甘油溶液中的α-乳清蛋白和β-乳球蛋白的葡萄糖糖基化
研究了甘油基液体系统中50°C下α-乳白蛋白和β-乳球蛋白的葡萄糖糖基化作用,以评估水分活度对甘油基糖基化和位点特异性的影响。使用邻苯二甲醛(OPA)方法以及超高效液相色谱结合电喷雾电离质谱(UPLC-ESI-MS)分析来确定反应过程中的糖基化程度。糖基化位点通过独立于数据的采集LC-MS(LC-MS E)。通过PyMOL获得的表面电势和通过圆二色性(CD)确定的三级结构可用于辅助分析甘油基质中的糖基化位点特异性。甘油溶液的水活度与甘油浓度负相关。结果表明,在最初的48小时内,甘油基质中的初始糖化率与线性方程拟合。不管蛋白质的天然结构如何,随着甘油浓度的增加,即水活度的降低,糖基化反应加速。尽管在不同的水分活度下,糖基化位点在相似的DSP上是相同的,分别在α-乳白蛋白和β-乳球蛋白中检测到4个和7个位点。但是,与水基基质中的糖基化位点相比,糖基化的位点特异性受甘油基质的影响,具体取决于蛋白质的天然结构。在分布于蛋白质表面的反应位点上,特别是在具有正电势的区域中,容易发生糖基化。
更新日期:2018-09-19
中文翻译:

甘油溶液中的α-乳清蛋白和β-乳球蛋白的葡萄糖糖基化
研究了甘油基液体系统中50°C下α-乳白蛋白和β-乳球蛋白的葡萄糖糖基化作用,以评估水分活度对甘油基糖基化和位点特异性的影响。使用邻苯二甲醛(OPA)方法以及超高效液相色谱结合电喷雾电离质谱(UPLC-ESI-MS)分析来确定反应过程中的糖基化程度。糖基化位点通过独立于数据的采集LC-MS(LC-MS E)。通过PyMOL获得的表面电势和通过圆二色性(CD)确定的三级结构可用于辅助分析甘油基质中的糖基化位点特异性。甘油溶液的水活度与甘油浓度负相关。结果表明,在最初的48小时内,甘油基质中的初始糖化率与线性方程拟合。不管蛋白质的天然结构如何,随着甘油浓度的增加,即水活度的降低,糖基化反应加速。尽管在不同的水分活度下,糖基化位点在相似的DSP上是相同的,分别在α-乳白蛋白和β-乳球蛋白中检测到4个和7个位点。但是,与水基基质中的糖基化位点相比,糖基化的位点特异性受甘油基质的影响,具体取决于蛋白质的天然结构。在分布于蛋白质表面的反应位点上,特别是在具有正电势的区域中,容易发生糖基化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号