当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic analysis of the steam reforming of ethanol over Ni/SiO2 for the elucidation of metal-dominated reaction pathways
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1039/c8re00145f Marinela D. Zhurka 1, 2, 3, 4, 5 , Angeliki A. Lemonidou 6, 7, 8, 9, 10 , James A. Anderson 1, 2, 3, 4, 5 , Panagiotis N. Kechagiopoulos 1, 2, 3, 4, 5
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1039/c8re00145f Marinela D. Zhurka 1, 2, 3, 4, 5 , Angeliki A. Lemonidou 6, 7, 8, 9, 10 , James A. Anderson 1, 2, 3, 4, 5 , Panagiotis N. Kechagiopoulos 1, 2, 3, 4, 5
Affiliation
|
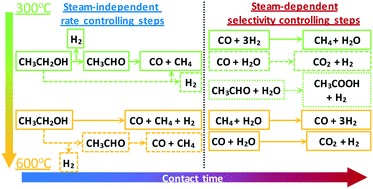
Hydrogen production via steam reforming of biomass-derived ethanol is a promising environmental alternative to the use of fossil fuels and a means of clean power generation. A kinetic study of ethanol steam reforming (ESR) is presented, where the effect of temperature, space time and partial pressure of reactants is investigated over a wide range in a fixed-bed reactor over a Ni/SiO2 catalyst. The order of the reaction was found to be 0.5 in ethanol and almost zero in water, indicating a steam-independent rate-limiting step, while an apparent activation energy of 48 kJ mol−1 was obtained. Identification of primary and secondary products revealed the reaction mechanism to be strongly affected by temperature with results suggesting the existence of two alternate pathways being active, one involving acetaldehyde and one an ethanol decomposition derived surface intermediate. Below 450 °C, ethanol decomposition and dehydrogenation were found to be dominant, whereas at higher temperatures secondary methane steam reforming (MSR) and water-gas shift (WGS) reactions became enhanced. An excess of water was able to promote the WGS reaction and suppress the methanation reaction even at 400 °C. Time-on-stream studies at 500 °C revealed Ni/SiO2 to have a good balance between stability, activity and selectivity in ESR. Temperature-programmed oxidation (TPO) and hydrogenation (TPH) analyses indicated that the carbonaceous deposits were graphitic in nature, suggesting the presence of filamentous coke.
中文翻译:

Ni / SiO 2上乙醇水蒸气重整以阐明金属主导反应路径的 动力学分析
通过蒸汽重整生物质衍生的乙醇来生产氢,是使用化石燃料和清洁发电的一种有前途的环境替代方案。提出了乙醇蒸汽重整(ESR)的动力学研究,其中在固定床反应器中Ni / SiO 2催化剂上,研究了温度,时空和反应物分压的影响。发现反应的顺序在乙醇中为0.5,在水中几乎为零,这表明了与蒸汽无关的限速步骤,而表观活化能为48 kJ mol -1获得了。初级产物和次级产物的鉴定揭示了反应机理受温度的强烈影响,结果表明存在两种交替的途径是活跃的,一种途径涉及乙醛,一种途径是乙醇分解衍生的表面中间体。在450°C以下,发现乙醇分解和脱氢占主导地位,而在较高温度下,二次甲烷蒸汽重整(MSR)和水煤气变换(WGS)反应得到增强。过量的水甚至在400℃下也能促进WGS反应并抑制甲烷化反应。在500°C下的时间流研究表明Ni / SiO 2在ESR的稳定性,活性和选择性之间保持良好的平衡。程序升温氧化(TPO)和氢化(TPH)分析表明,碳质沉积物的性质为石墨,表明存在丝状焦炭。
更新日期:2018-09-18
中文翻译:

Ni / SiO 2上乙醇水蒸气重整以阐明金属主导反应路径的 动力学分析
通过蒸汽重整生物质衍生的乙醇来生产氢,是使用化石燃料和清洁发电的一种有前途的环境替代方案。提出了乙醇蒸汽重整(ESR)的动力学研究,其中在固定床反应器中Ni / SiO 2催化剂上,研究了温度,时空和反应物分压的影响。发现反应的顺序在乙醇中为0.5,在水中几乎为零,这表明了与蒸汽无关的限速步骤,而表观活化能为48 kJ mol -1获得了。初级产物和次级产物的鉴定揭示了反应机理受温度的强烈影响,结果表明存在两种交替的途径是活跃的,一种途径涉及乙醛,一种途径是乙醇分解衍生的表面中间体。在450°C以下,发现乙醇分解和脱氢占主导地位,而在较高温度下,二次甲烷蒸汽重整(MSR)和水煤气变换(WGS)反应得到增强。过量的水甚至在400℃下也能促进WGS反应并抑制甲烷化反应。在500°C下的时间流研究表明Ni / SiO 2在ESR的稳定性,活性和选择性之间保持良好的平衡。程序升温氧化(TPO)和氢化(TPH)分析表明,碳质沉积物的性质为石墨,表明存在丝状焦炭。



























 京公网安备 11010802027423号
京公网安备 11010802027423号