Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Significance of Lone Pair/Lone Pair and Lone Pair/Bond Pair Repulsions in the Cation Affinity and Lewis Acid/Lewis Base Interactions
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1021/acsomega.8b01644 Younes Valadbeigi 1 , Jean-François Gal 2
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1021/acsomega.8b01644 Younes Valadbeigi 1 , Jean-François Gal 2
Affiliation
|
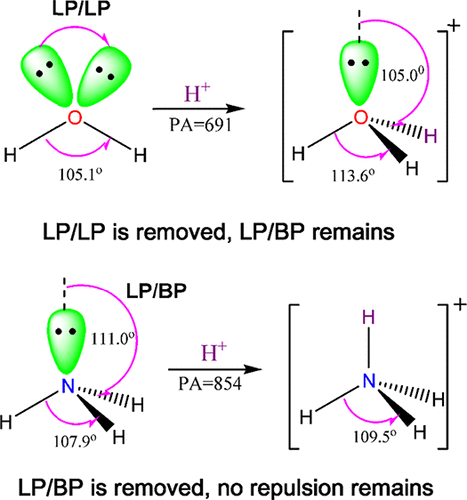
Interaction of H2O, H2S, H2Se, NH3, PH3, and AsH3 with cations H+, CH3+, Cu+, Al+, Li+, Na+, and K+ was studied from the energetic and structural viewpoint using B3LYP/6-311++G(d,p) method. The charge transfer from the Lewis bases to the cations reduces lone pair/lone pair (LP/LP) repulsion in H2O, H2S, and H2Se and LP/bond pair (LP/BP) repulsion in NH3, PH3, and AsH3. In parallel, changes in the H–M–H angles (M = O, S, Se, N, P, and As) are observed. The change in the H–M–H angle during the interactions was proportional to the amount of charge transferred from the bases to the cations and electron density (ρ) at the molecule/cation bond critical point. Also, the opposite trend for proton affinities of these two families, that is, NH3 > PH3 > AsH3 and H2O < H2S < H2Se, was interpreted on the basis of LP/BP repulsion in their neutral and protonated forms. Interaction of the Lewis bases with neutral Lewis acids including BeH2, BeF2, and BH3 was studied energetically and structurally. The calculated energies for interactions of H2O and NH3 with BeH2, BeF2, and BH3 are larger than the corresponding values for H2S, H2Se, PH3, and AsH3. This difference was interpreted on the basis of the lower stability of H2O and NH3 because of large LP/LP and LP/BP repulsion in H2O and LP/BP repulsion in NH3.
中文翻译:

孤对/孤对和孤对/键对的排斥在阳离子亲和力和路易斯酸/路易斯碱相互作用中的意义
研究了H 2 O,H 2 S,H 2 Se,NH 3,PH 3和AsH 3与阳离子H +,CH 3 +,Cu +,Al +,Li +,Na +和K +的相互作用。使用B3LYP / 6-311 ++ G(d,p)方法获得的能量和结构视点。从路易斯碱的电荷转移到阳离子减少孤对/孤对(LP / LP)排斥H中2 O,H 2 S,和H 2 Se和LP /键对(LP / BP)斥力在NH 3, PH值3和AsH 3。平行地,观察到H–M–H角度的变化(M = O,S,Se,N,P和As)。在相互作用期间,H–M–H角的变化与从碱转移到阳离子的电荷量和分子/阳离子键临界点的电子密度(ρ)成正比。同样,这两个家族的质子亲和力相反的趋势,即NH 3 > PH 3 > AsH 3和H 2 O <H 2 S <H 2 Se,是根据中性的LP / BP排斥来解释的。和质子化形式。Lewis碱与中性Lewis酸(包括BeH 2,BeF 2和BH 3)的相互作用在精力和结构上进行了研究。H 2 O和NH 3与BeH 2,BeF 2和BH 3相互作用的计算能量大于H 2 S,H 2 Se,PH 3和AsH 3的相应值。这种差异被解释H的低稳定性的基础上,2 O和NH 3,因为大型LP / LP和LP / BP斥力在H的2 O和LP / BP斥力在NH 3。
更新日期:2018-09-18
中文翻译:

孤对/孤对和孤对/键对的排斥在阳离子亲和力和路易斯酸/路易斯碱相互作用中的意义
研究了H 2 O,H 2 S,H 2 Se,NH 3,PH 3和AsH 3与阳离子H +,CH 3 +,Cu +,Al +,Li +,Na +和K +的相互作用。使用B3LYP / 6-311 ++ G(d,p)方法获得的能量和结构视点。从路易斯碱的电荷转移到阳离子减少孤对/孤对(LP / LP)排斥H中2 O,H 2 S,和H 2 Se和LP /键对(LP / BP)斥力在NH 3, PH值3和AsH 3。平行地,观察到H–M–H角度的变化(M = O,S,Se,N,P和As)。在相互作用期间,H–M–H角的变化与从碱转移到阳离子的电荷量和分子/阳离子键临界点的电子密度(ρ)成正比。同样,这两个家族的质子亲和力相反的趋势,即NH 3 > PH 3 > AsH 3和H 2 O <H 2 S <H 2 Se,是根据中性的LP / BP排斥来解释的。和质子化形式。Lewis碱与中性Lewis酸(包括BeH 2,BeF 2和BH 3)的相互作用在精力和结构上进行了研究。H 2 O和NH 3与BeH 2,BeF 2和BH 3相互作用的计算能量大于H 2 S,H 2 Se,PH 3和AsH 3的相应值。这种差异被解释H的低稳定性的基础上,2 O和NH 3,因为大型LP / LP和LP / BP斥力在H的2 O和LP / BP斥力在NH 3。











































 京公网安备 11010802027423号
京公网安备 11010802027423号