当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Blood-Brain Barrier Transport, Plasma Pharmacokinetics, and Neuropathology Following Chronic Treatment of the Rhesus Monkey with a Brain Penetrating Humanized Monoclonal Antibody Against the Human Transferrin Receptor
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00730 William M. Pardridge 1 , Ruben J. Boado 1 , Daniel J. Patrick 2 , Eric Ka-Wai Hui 1 , Jeff Zhiqiang Lu 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00730 William M. Pardridge 1 , Ruben J. Boado 1 , Daniel J. Patrick 2 , Eric Ka-Wai Hui 1 , Jeff Zhiqiang Lu 1
Affiliation
|
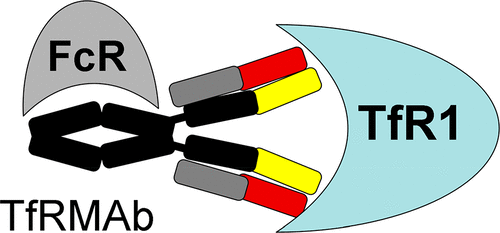
A monoclonal antibody (mAb) against the blood-brain barrier (BBB) transferrin receptor (TfR) is a potential agent for delivery of biologic drugs to the brain across the BBB. However, to date, no TfRMAb has been tested with chronic dosing in a primate model. A humanized TfRMAb against the human (h) TfR1, which cross reacts with the primate TfR, was genetically engineered with high affinity (ED50 = 0.18 ± 0.04 nM) for the human TfR type 1 (TfR1). For acute dosing, the hTfRMAb was tritiated and injected intravenously (IV) in the Rhesus monkey, which confirmed rapid delivery of the humanized hTfRMAb into both brain parenchyma, via transport across the BBB, and into cerebrospinal fluid (CSF), via transport across the choroid plexus. For chronic dosing, a total of 8 adult Rhesus monkeys (4 males, 4 females) were treated twice weekly for 4 weeks with 0, 3, 10, or 30 mg/kg of the humanized hTfRMAb via a 60 min IV infusion for a total of 8 doses prior to euthanasia and microscopic examination of brain and peripheral organs. A pharmacokinetics analysis showed the plasma clearance of the hTfRMAb in the primate was nonlinear, and plasma clearance was increased over 20-fold with chronic treatment of the low dose, 3 mg/kg, of the antibody. Chronic treatment of the primates with the 30 mg/kg dose caused anemia associated with suppressed blood reticulocytes. Immunohistochemistry of terminal brain tissue showed microglia activation, based on enhanced IBA1 immuno-staining, in conjunction with astrogliosis, based on increased GFAP immuno-staining. Moderate axonal/myelin degeneration was observed in the sciatic nerve. Further studies need to be conducted to determine if this neuropathology is induced by the antibody effector function, or is an intrinsic property of targeting the TfR in brain. The results indicate that chronic treatment of Rhesus monkeys with a humanized hTfRMAb may have a narrow therapeutic index, with associated toxicity related to microglial activation and astrogliosis of the brain.
中文翻译:

用脑穿透性人源化抗人转铁蛋白受体单克隆抗体对恒河猴进行慢性治疗后的血脑屏障运输,血浆药代动力学和神经病理学
抗血脑屏障(BBB)转铁蛋白受体(TfR)的单克隆抗体(mAb)是通过BBB向大脑输送生物药物的潜在药物。然而,迄今为止,尚未在灵长类动物模型中用长期给药对TfRMAb进行过测试。与人(h)TfR1发生人源化的TfRMAb与灵长类TfR发生交叉反应,并以高亲和力(ED50 = 0.18±0.04 nM)对人TfR 1型(TfR1)进行了基因改造。对于急性给药,将hTfRMAb tri化并静脉内(IV)注射到恒河猴中,这证实了人源化hTfRMAb通过跨BBB的转运迅速进入脑实质,并通过跨BBB的转运迅速进入脑脊液(CSF)。脉络丛。对于长期给药,每周对总共8只成年恒河猴(4只雄性,4只雌性)进行两次治疗,持续4周,其中0,在对安乐死和脑部和周围器官进行显微镜检查之前,通过60分钟的静脉输注60 mg,3、10或30 mg / kg的人源化hTfRMAb,共8剂。药代动力学分析表明,在灵长类动物中,hTfRMAb的血浆清除率是非线性的,并且通过长期治疗低剂量3 mg / kg抗体,血浆清除率增加了20倍以上。用30 mg / kg剂量的灵长类动物进行慢性治疗会导致与网状红细胞抑制相关的贫血。末梢脑组织的免疫组织化学显示,基于增强的IBA1免疫染色的小胶质细胞活化,以及基于GFAP免疫染色的星形胶质增生。在坐骨神经中观察到中度轴突/髓鞘变性。需要进行进一步的研究以确定这种神经病理是由抗体效应子功能诱导的,还是靶向于脑中TfR的内在特性。结果表明,用人源化的hTfRMAb长期治疗恒河猴可能具有较窄的治疗指数,并伴有与小胶质细胞活化和大脑星形胶质增生相关的毒性。
更新日期:2018-09-18
中文翻译:

用脑穿透性人源化抗人转铁蛋白受体单克隆抗体对恒河猴进行慢性治疗后的血脑屏障运输,血浆药代动力学和神经病理学
抗血脑屏障(BBB)转铁蛋白受体(TfR)的单克隆抗体(mAb)是通过BBB向大脑输送生物药物的潜在药物。然而,迄今为止,尚未在灵长类动物模型中用长期给药对TfRMAb进行过测试。与人(h)TfR1发生人源化的TfRMAb与灵长类TfR发生交叉反应,并以高亲和力(ED50 = 0.18±0.04 nM)对人TfR 1型(TfR1)进行了基因改造。对于急性给药,将hTfRMAb tri化并静脉内(IV)注射到恒河猴中,这证实了人源化hTfRMAb通过跨BBB的转运迅速进入脑实质,并通过跨BBB的转运迅速进入脑脊液(CSF)。脉络丛。对于长期给药,每周对总共8只成年恒河猴(4只雄性,4只雌性)进行两次治疗,持续4周,其中0,在对安乐死和脑部和周围器官进行显微镜检查之前,通过60分钟的静脉输注60 mg,3、10或30 mg / kg的人源化hTfRMAb,共8剂。药代动力学分析表明,在灵长类动物中,hTfRMAb的血浆清除率是非线性的,并且通过长期治疗低剂量3 mg / kg抗体,血浆清除率增加了20倍以上。用30 mg / kg剂量的灵长类动物进行慢性治疗会导致与网状红细胞抑制相关的贫血。末梢脑组织的免疫组织化学显示,基于增强的IBA1免疫染色的小胶质细胞活化,以及基于GFAP免疫染色的星形胶质增生。在坐骨神经中观察到中度轴突/髓鞘变性。需要进行进一步的研究以确定这种神经病理是由抗体效应子功能诱导的,还是靶向于脑中TfR的内在特性。结果表明,用人源化的hTfRMAb长期治疗恒河猴可能具有较窄的治疗指数,并伴有与小胶质细胞活化和大脑星形胶质增生相关的毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号