当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Safety Evaluation of the Copper-Mediated Cross-Coupling of 2-Bromopyridines with Ethyl Bromodifluoroacetate
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1021/acs.oprd.8b00261 Qiang Yang 1 , Pablo J. Cabrera 1 , Xiaoyong Li 2 , Min Sheng 3 , Nick X. Wang 4
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1021/acs.oprd.8b00261 Qiang Yang 1 , Pablo J. Cabrera 1 , Xiaoyong Li 2 , Min Sheng 3 , Nick X. Wang 4
Affiliation
|
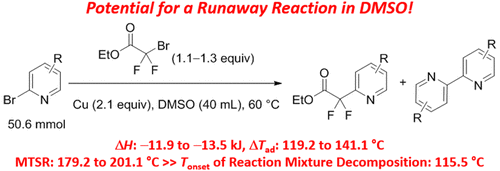
The potential safety hazards associated with the copper-mediated cross-coupling of 2-bromopyridines with ethyl bromodifluoroacetate were evaluated. Thermal stability evaluation of the postreaction mixture of 50.6 mmol of 2-bromopyridine with 1.3 equiv of ethyl bromodifluoroacetate in the presence of 2.1 equiv of copper in 40 mL of dimethyl sulfoxide (DMSO) indicated a significant decomposition event with an onset temperature of 115.5 °C by accelerating rate calorimetry, which was significantly lower than that of neat DMSO. In contrast, the reaction mixture in N,N-dimethylformamide did not show any exothermic decomposition up to 400 °C by differential scanning calorimetry. Reaction calorimetry evaluation of this reaction in DMSO revealed a heat output (ΔH) of −13.5 kJ and an adiabatic temperature rise (ΔTad) of 129.5 °C, resulting in a maximum temperature of a synthesis reaction (MTSR) of 189.5 °C. The predicted heat of reaction using density functional theory with the BLYP functional was in good agreement with the experimental data. The scope studies with a variety of substituted 2-bromopyridines revealed similar magnitudes of ΔH and ΔTad compared to 2-bromopyridine when reacted at the same concentration. In all of the studied cases, the MTSR was significantly higher than the onset temperature of reaction mixture decomposition, indicating that in the absence of active cooling the system could quickly trigger the decomposition of the reaction mixture, resulting in a runaway reaction.
中文翻译:

铜介导的2-溴吡啶与溴二氟乙酸乙酯的交叉偶联的安全性评估
评价了2-溴吡啶与溴代二氟乙酸乙酯的铜介导的交叉偶联相关的潜在安全隐患。在40 mL二甲基亚砜(DMSO)中存在2.1当量铜的情况下,对50.6 mmol 2-溴吡啶和1.3当量溴代二氟乙酸乙酯的后反应混合物进行热稳定性评估,表明发生了明显的分解事件,起始温度为115.5°C通过加速量热法,明显低于纯DMSO。相反,在N,N-二甲基甲酰胺中的反应混合物通过差示扫描量热法在400℃以下没有显示任何放热分解。在DMSO中对该反应的反应量热法评估显示出热量输出(ΔH-13.5 kJ)和绝热温升(ΔT ad)为129.5°C,导致合成反应(MTSR)的最高温度为189.5°C。使用具有BLYP功能的密度泛函理论预测的反应热与实验数据非常吻合。当以相同浓度反应时,与2-溴吡啶相比,用各种取代的2-溴吡啶进行的范围研究表明,ΔH和ΔT ad的幅值相近。在所有研究的情况下,MTSR均显着高于反应混合物分解的起始温度,这表明在没有主动冷却的情况下,系统会迅速触发反应混合物的分解,从而导致反应失控。
更新日期:2018-09-18
中文翻译:

铜介导的2-溴吡啶与溴二氟乙酸乙酯的交叉偶联的安全性评估
评价了2-溴吡啶与溴代二氟乙酸乙酯的铜介导的交叉偶联相关的潜在安全隐患。在40 mL二甲基亚砜(DMSO)中存在2.1当量铜的情况下,对50.6 mmol 2-溴吡啶和1.3当量溴代二氟乙酸乙酯的后反应混合物进行热稳定性评估,表明发生了明显的分解事件,起始温度为115.5°C通过加速量热法,明显低于纯DMSO。相反,在N,N-二甲基甲酰胺中的反应混合物通过差示扫描量热法在400℃以下没有显示任何放热分解。在DMSO中对该反应的反应量热法评估显示出热量输出(ΔH-13.5 kJ)和绝热温升(ΔT ad)为129.5°C,导致合成反应(MTSR)的最高温度为189.5°C。使用具有BLYP功能的密度泛函理论预测的反应热与实验数据非常吻合。当以相同浓度反应时,与2-溴吡啶相比,用各种取代的2-溴吡啶进行的范围研究表明,ΔH和ΔT ad的幅值相近。在所有研究的情况下,MTSR均显着高于反应混合物分解的起始温度,这表明在没有主动冷却的情况下,系统会迅速触发反应混合物的分解,从而导致反应失控。











































 京公网安备 11010802027423号
京公网安备 11010802027423号